Asthma is common among Australian children, with an estimated prevalence of 14%–16%.1 Over the past decade, hospitalisations and deaths related to asthma have decreased significantly.1 Although the use of potent inhaled corticosteroids has increased in Australia,1 as well as in New Zealand2 and Scotland,3 little is known of the changing profile of medication use among children with asthma.
Published studies of the pharmacoepidemiology of childhood asthma generally explore overall trends by collating state and national data on pharmacy prescriptions.4 Those that have examined children’s medication use have been cross-sectional studies relying on general practice data,5 or telephone surveys.6 These may permit more detailed analysis of medication use by children with asthma but do not allow us to examine time trends.
All school-entry children in the ACT undergo health screening in kindergarten, which includes a survey completed by parents (delivery and structure described elsewhere7). Since 1998, this survey has comprised a general health questionnaire and an 18-item questionnaire for children with respiratory symptoms. The respiratory questionnaire includes an item asking parents to identify (from a checklist of proprietary names of asthma drugs) medications taken by their child in the last year. Parents report the average frequency of use per week for each class of medication (preventers, relievers, symptom controllers, combination products and oral corticosteroids).
We analysed data on asthma medication use for children with parent-reported asthma for the years 2000–2005. Data from 1998 and 1999 were excluded because of inconsistencies in data collection instruments in those years. International Study of Asthma and Allergies in Childhood questions were used to determine the severity and pattern of asthma (infrequent, frequent or persistent).8 We assessed statistical significance by means of χ2 tests for trend.
To validate our findings, we examined Medicare Australia data on prescriptions for fluticasone-containing products, salbutamol nebules and cromoglycate in the ACT for children who turned 5 years of age before 1 May for the years 2002–2005. Medicare Australia withheld data on prescriptions for a medication if fewer than five were dispensed per year. Using mid-year age-specific population estimates as the denominator, we calculated defined daily doses per thousand 5-year-old children for these medications.9 For combination products, the principles outlined by the World Health Organization Collaborating Centre for Drug Statistics Methodology were followed to develop an overall defined daily dose per thousand 5-year-old children for fluticasone.10
The ACT Department of Health and Community Care Institutional Ethics Committee approved this study.
Response rates (shown in Box 1) to the general health questionnaire ranged from 85% to 89%. For children with parent-reported asthma, response rates to the question on asthma medications ranged from 91% to 97%.
The prevalence of parent-reported asthma ranged from 11% to 15% (Box 1), affecting at least 430 children annually. The children’s ages ranged from 4 to 6 years (median, 5 years). Sixty per cent were boys. A third of children with asthma were classified as having mild asthma, and 30% were classified as having severe asthma. Twelve per cent of children were reported to have needed admission to intensive care or a high dependency unit because of their asthma.
Box 2 shows that inhaled corticosteroid use remained stable, despite decreases in the use of budesonide and beclomethasone, and a threefold increase in fluticasone use. Use of all fluticasone-containing products increased from 11% to 46% of all children with asthma (χ2 for trend, 112; P < 0.001).
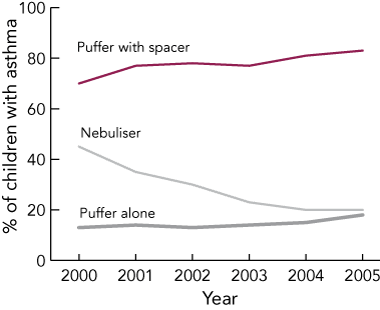
Box 3 shows that reported use of spacers increased over the study period from 70% to 83% of children with asthma (χ2 for trend, 23; P < 0.001), while reported nebuliser use fell from 45% to 20% of children with asthma (χ2 for trend, 106; P < 0.001). The frequency of medication use remained stable over the 6 years, with 53% of children taking preventer medications (inhaled corticosteroids, mast cell stabilisers, montelukast) at least 4 days per week.
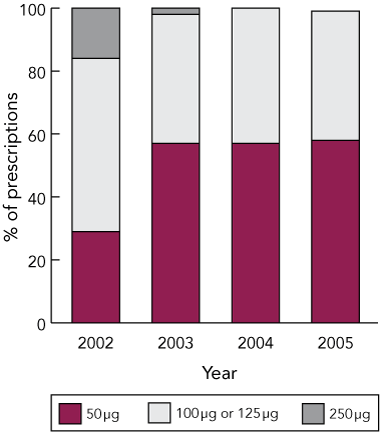
Medicare Australia data (Box 4) showed increases in the total number of prescriptions dispensed for fluticasone-containing products from 2002 to 2005, but a decrease in the number of defined daily doses per thousand 5-year-old children. Over the same period, numbers of prescriptions for salbutamol nebules and cromoglycate decreased. Medicare Australia data also showed a shift to less potent forms of fluticasone from 2003 (Box 5).
Our study period coincides with an important event in asthma management in Australia — the Asthma Management Program, which was announced in the 2001–02 federal budget, and emphasised evidence-based asthma management.1 Some of the volatility in the use of asthma medications and delivery devices may reflect improved awareness among doctors and patients of best practice. The decline in nebuliser use may indicate improved confidence among parents in the use of spacer devices, reflecting changes in emergency department and specialist practice, and instruction in these techniques by community asthma educators. The fall in the use of ipratropium bromide probably reflects the decline in nebuliser use, as this drug is sold in nebules as well as in a metered aerosol. Declines in the use of cromoglycate and nedocromil are part of an international trend, with heightened emphasis on the use of inhaled corticosteroids, and doubts about the effectiveness of sodium cromoglycate.11
Although corticosteroid use remained largely unchanged, there was a marked increase in the use of fluticasone. Use of inhaled corticosteroids is generally held to be a marker of good asthma management (a recent meta-analysis found that, at recommended doses, they pose no risk to long-term statural growth, cortisol levels, and bone mineral density,12 although inhaled budesonide and, to a lesser extent, fluticasone do slow growth velocity13). Nevertheless, potent corticosteroids like fluticasone may be unsafe in children if effective back-titration is not undertaken when appropriate. In New Zealand, the introduction of fluticasone led to a near doubling of the equipotent dose of inhaled corticosteroid given to children aged under 5 years, because of its increased potency.3 In Australia, the Pharmaceutical Benefits Scheme moved to contain the use of high-dose fluticasone by limiting the number of repeat prescriptions for preparations containing 250 μg or 500 μg of fluticasone. Our analysis of Medicare data indicated that, since 2003, more ACT children who use fluticasone have been prescribed lower doses.
Although reported use of salmeterol was stable across the study period, there was a significant increase in use of the medication containing a combination of salmeterol and fluticasone, with a fifth of children with asthma using this product in 2005. Combination products have a role in managing children who require at least one dose of β2 agonist per day,14 although the evidence supporting the efficacy of fixed-dose combinations for children is not substantial.15 Because of concerns about an increased risk of unexplained death with salmeterol,16 the Therapeutic Goods Administration has recommended that long-acting β2 agonists only be used in combination with inhaled corticosteroids.17 However, recent reports suggest that, even in combination with corticosteroids, long-acting β2 agonists may pose an increased risk of asthma death, particularly in genetically predisposed subpopulations.18
1 Study and sample population response rates, Australian Capital Territory kindergarten health screening data, 2000–2005
2 Trends in reported medication use in the preceding 12 months for Australian Capital Territory school-entry children with parent-reported asthma, 2000–2005 (ACT kindergarten health screening data)
3 Trends in the use of delivery devices for asthma medication among Australian Capital Territory school-entry children, 2000–2005 (ACT kindergarten health screening data)
Received 14 November 2006, accepted 19 April 2007
- Christine B Phillips1
- Helen Toyne1
- Karen Ciszek1
- Robyn G Attewell2
- Marjan Kljakovic1
- 1 Academic Unit of General Practice and Community Health, Australian National University, Canberra, ACT.
- 2 Covance Pty Ltd, Canberra, ACT.
We are grateful to the maternal and child nurses from ACT Health who undertook the kindergarten health screening, Dr Clare McGuiness for advice, and Ms Maxine Robinson and staff of the Drug Utilisation Sub-Committee of the Pharmaceutical Benefits Advisory Committee, Australian Government Department of Health and Ageing for assistance with accessing Medicare Australia data.
Covance Pty Ltd has ongoing consulting agreements with Boehringer Ingelheim, AstraZeneca and GlaxoSmithKline Australia. Robyn Attewell has been involved in research in primary care and clinical settings under these contracts. Her contribution to this article was not related to these contracts, and was under a separate consulting contract with ACT Health and Community Care.
- 1. Australian Institute of Health and Welfare Australian Centre for Asthma Monitoring. Asthma in Australia 2005. AIHW Asthma series No. 2. Canberra: AIHW, 2005. (AIHW Cat. No. ACM 6.) http://www.aihw.gov.au/publications/index.cfm/title/10158 (accessed May 2007).
- 2. Johansson M, Hall J, Reith D, et al. Trends in the use of inhaled corticosteroids for childhood asthma in New Zealand. Eur J Clin Pharmacol 2003; 59: 483-487.
- 3. Boyter AC, Steinke DT. Changes in prescribing of inhaled corticosteroids (1999–2002) in Scotland. Pharmacoepidemiol Drug Saf 2005; 14: 203-209.
- 4. Robertson J, Fryer JL, O’Connell DL, et al. Limitations of Health Insurance Commission (HIC) data for deriving prescribing indicators. Med J Aust 2002; 176: 419-424. <MJA full text>
- 5. Britt H, Miller GC, Knox S, et al. General practice activity in Australia 2004–05. General Practice Series No. 18. Canberra: Australian Institute of Health and Welfare, 2005. (AIHW Cat. No. GEP 18.) http://www.aihw.gov.au/publications/index.cfm/title/10189 (accessed Jun 2007).
- 6. Mommers M, Swaen GM, Weishoff-Houben M, et al. Differences in asthma diagnosis and medication use in children living in Germany and the Netherlands. Prim Care Respir J 2005; 14: 31-37.
- 7. Phillips CB, Yates R, Glasgow NJ, et al. Improving response rates to primary and supplementary questionnaires by changing response and instruction burden: cluster randomised trial. Aust N Z J Public Health 2005; 29: 457-460.
- 8. Ellwood P, Asher MI, Beasley R, on behalf of the International Study of Asthma and Allergies in Childhood Steering Committee and the ISAAC Phase Three Study Group. ISAAC phase three manual. Auckland: ISAAC, 2000. http://isaac.auckland.ac.nz/Phasethr/Manual.pdf (accessed May 2007).
- 9. Australian Bureau of Statistics. Population by age and sex, Australian states and territories, 2006. Canberra: ABS, 2006. (ABS Cat. No. 3201.0.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3201.0Jun%202006? OpenDocument (accessed May 2007).
- 10. World Health Organization Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. Oslo: WHOCC for Drug Statistics Methodology, January 2006.
- 11. Van der Wouden JC, Tasche MJA, Bernsen RMD, et al. Sodium cromoglycate for asthma in children. Cochrane Database Syst Rev 2003; (3): CD002173. DOI: 10.1002/14651858.CD002173 http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002173/frame.html (accessed Jun 2007).
- 12. Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf 2006; 29: 599-612.
- 13. Ferguson AC, Van Bever HP, Teper AM, et al. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respir Med 2007; 101: 118-129.
- 14. Asthma management handbook 2006. Melbourne: National Asthma Council Australia, 2006. http://www.NationalAsthma.org.au/publications/amh/amhcont.htm (accessed May 2007).
- 15. Bisgaard H, Le Roux P, Bjamer D, et al. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest 2006; 130: 1733-1744.
- 16. Nelson HS, Weiss SC, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129: 15-26. [Erratum: Chest 2006; 129: 1393.]
- 17. Janson C, de Marco R, Accordini S, et al. Changes in the use of anti-asthmatic medication in an international cohort. Eur Respir J 2005; 26: 1047-1055.
- 18. Martinez FD. Safety of long-acting beta-agonists — an urgent need to clear the air. N Engl J Med 2005; 353: 2637-2639.





Abstract
Objective: To analyse trends in asthma medications used by school-entry children whose parents report they have asthma.
Design and setting: Annual cross-sectional study of all school-entry children (about 4400 each year) in the Australian Capital Territory in 2000–2005, by means of a questionnaire for parents on child health status and medication use; and a cross-sectional study of asthma prescriptions for children aged 5 years obtained from the Medicare Australia database for 2002–2005.
Participants: All school-entry children in the ACT with parent-reported asthma (numbers in the years 2000–2005 ranged between 435 and 589).
Main outcome measures: Changes in the use of different medications; changes in delivery devices for asthma; changes in the potency of inhaled fluticasone.
Results: Response rates to kindergarten health screening were in the range 85%–89% for 2000–2005. Parent-reported asthma prevalence ranged from 11% to 15%. Each year, around 35% of children with asthma (age range, 4–6 years) used inhaled corticosteroids. An increase in the use of fluticasone (from 11% to 33% of children with asthma) was offset by decreases in beclomethasone use (from 14% to 3%) and budesonide (from 14% to 4%). Use of cromoglycate and nedocromil fell from 46% to 16%. Nebuliser use decreased (from 45% to 20%), while the use of spacer devices increased (from 70% to 83%). Use of combined salmeterol/fluticasone increased from 8% (in 2002) to 20% (in 2005) of children with parent-reported asthma. These trends were mirrored in Medicare Australia data for 5-year-old children in the ACT.
Conclusions: There was marked volatility in the types of asthma medication used over the 6 years. Reciprocal trends leading to increased use of spacers and decreased use of nebulisers are in accord with national guidelines for better asthma management. The increasing use of products containing a combination of salmeterol and fluticasone requires ongoing monitoring.