Before 2002, observational studies (Level III-2 and Level III-3 evidence, see Box 11) were mostly of women who commenced hormone replacement therapy (HRT) for symptom control near menopause. These studies suggested that taking long-term HRT reduced cardiovascular and fracture risk, but increased the risk of breast cancer and thromboembolism. In 2002, the initial results of a long-term randomised controlled trial (Level II) of HRT (Women’s Health Initiative [WHI]) showed that, after 5 years of combined oestrogen and progestogen therapy in a relatively asymptomatic, older population, commencing therapy on average about 13 years after menopause, there was a significant reduction in fractures, no overall cardiovascular benefit, and an increased occurrence of breast cancer and thromboembolism.2
The media reaction to this first look at the WHI data encouraged up to two-thirds of users of HRT to stop the therapy, often without medical consultation.3 Various professional and non-professional bodies rather too quickly issued edicts, based on the WHI report, saying that HRT should only be used at the lowest dose for the shortest possible time in women with severe symptoms.4
Recent analyses of the WHI data, other randomised controlled trials, and observational and animal studies have now unified much of the data on HRT and greatly changed the risk–benefit ratio for most women who commence HRT for symptom control around menopause. It is mostly good news (Box 2).
There are now strong data in support of the “critical therapeutic window” hypothesis that oestrogen is cardioprotective if initiated around menopause when there are still vascular oestrogen receptors responsive to exogenous HRT.5-7 HRT administered near menopause appears to reduce the progression of atherosclerotic plaque, but if administered many years after menopause it is not beneficial and may sometimes disrupt established plaque with adverse outcomes.
A meta-analysis of randomised trials (Level I) has shown a statistically and clinically significant 39% reduction in cardiac events in the treatment groups, compared with the placebo control groups, when HRT is initiated in women under 60 years of age (odds ratio [OR], 0.68; 95% CI, 0.48–0.96), but this cardioprotective effect was not seen in women starting HRT after age 60 years (OR, 1.03; 95% CI, 0.91–1.16).8 When HRT is first taken many years after menopause, there is an increase in cardiac events during the first year of therapy (hazard ratio [HR], 1.47; 95% CI, 1.12–1.92).8 Subsequent cardiac morbidity is reduced after taking HRT for 2 years in these older women (HR, 0.79; 95% CI, 0.67–0.93).8 All-cause mortality in younger HRT users compared with placebo is also significantly reduced (HR, 0.61; 95% CI, 0.39–0.95).9 Currently, data from Level II trials in women near menopause suggest that oestrogen-only regimens may offer greater cardioprotection than some combined regimens, but more research is needed on the timing and type of progestogen therapy in combined regimens.7,10
The two long-term Level II trials of HRT (WHI and WISDOM –Women’s International Study of long Duration Oestrogen after Menopause) enrolled women who were on average 13–14 years post-menopause, because the outcomes being measured are more prevalent at older ages. The populations in these trials were unrepresentative of symptomatic women who start taking HRT near menopause. Although the WHI alone did not have sufficient power to allow subanalyses of cardiac events in the 8832 women under age 60 years in the two HRT trial arms, data from the WHI now suggest a cardioprotective effect in women commencing HRT near menopause, especially when oestrogen-only regimens are used.6-8,10
Before the WHI, observational studies (Level III-2 and Level III-3) had suggested an increased relative risk of breast cancer with long-term combined HRT (cHRT) of 1.53 after a median of 8 years.11 The WHI actually reported half this risk, with a relative risk after 5.6 years of cHRT of 1.26 (adjusted 95% CI, 0.83–1.92).2 However, media reports often highlighted the relative risk without explaining the absolute risk. The absolute increased risk found in the WHI was 8 per 10 000 women-years, or less than 0.1% per annum. Systematic reviews of all the Level II and III-2 data now suggest that, in women taking combined HRT, there is an increased risk of breast cancer of 4 per 10 000 women-years, or 2 per 1000 women after 5 years.12-14 Some groups have preferred to use worst-case scenario statistics derived from observational data, including the Million Women Study (Level III-3).15 Observational data may increase the real risk and benefits (due to selection and detection biases), whereas intention-to-treat analyses of randomised controlled trials may reduce the real risk and benefits attributable to the therapy if non-compliance rates are high. Both methods have their merits and demerits, but Level II evidence is usually regarded as stronger evidence than Level III-2 evidence!
Another way to look at risk is to compare the increased relative risk (RR) of breast cancer found in the WHI (1.26) with other common risks. Thus, this relative risk is similar to the breast cancer risk in a woman with late menopause at age 55 or more (RR, 1.22); in a woman who has three alcoholic drinks per day (RR, 1.4); or in a nulliparous woman (RR, 1.67).16 Subsequent analysis of the WHI data showed that there was no significant increase in breast cancer among those who initiated cHRT for the first time during the 7 years of the WHI.17
Even better news from the WHI in relation to breast cancer came when outcomes of the oestrogen-only arm in women who had had a hysterectomy showed an almost statistically significant reduction in breast cancer (HR, 0.77; 95% CI, 0.59–1.01).18 These results challenge many of the beliefs about oestrogen and breast cancer, but may incriminate use of systemic progestogen. Data on regimens that avoid the undoubted risk of long-term cHRT (eg, tibolone or intrauterine progestogen and systemic oestrogen) are awaited. Observational data (Level III-3) have suggested that more than 20 years of oestrogen-only therapy may increase breast cancer rates.19
Thromboembolism remains the main short-term serious risk of taking HRT. The risk of thromboembolism in women taking oral HRT appears to be greatest in the first year or two of use and is highest in those with thrombophilia and/or obesity.20 The absolute risk varies with the individual risk for thromboembolism. The risk increases with age at initiation of HRT.20 In the future, general screening for thrombophilia may become a cost-effective proposition; currently, screening women with clinical risk factors for thromboembolism may be justified. Non-oral routes of oestrogen intake, adding micronised progesterone, or pregnane-derived progestogens in women with an intact uterus have not been associated with thromboembolic risk (Level III-2), but no long-term randomised trials have been performed.21
The expected one-third reduction in fractures (hip, spine and overall) seen in observational studies was confirmed by the WHI (cHRT — HR, 0.76; 95% CI, 0.69–0.92; oestrogen replacement therapy — HR, 0.70; 95% CI, 0.59–0.83).2,18 Importantly, this reduction was seen in a population not screened for osteoporosis. HRT remains a cost-efficient and relatively safe option for the prevention of fractures when initiated before age 60 years in women with osteoporosis, who often also have menopausal symptoms.22,23 Such women may have few other cost-efficient therapeutic options, and this indication for HRT needs to be revisited now the risks of HRT (especially low-dose, oestrogen-only regimens) have been recalculated.
The effect of HRT on cognitive function is likely to remain controversial because a long-term trial from the time of menopause will probably be impossible. Observational studies (Level III-2) support the critical therapeutic window hypothesis in which HRT use from near menopause shows greater benefit for cognitive function than commencing HRT many years after menopause.24 Among women in the WHI trial who commenced HRT when over 65 years of age, a slight detriment in cognitive function was found in this older group.25 The Cache County observational study noted a 59% reduction in dementia in women taking HRT from early menopause and for more than 10 years.26 Other Level III-3 studies, which have not distinguished between early or late initiation of HRT, have not reported a consistent cognitive benefit.
In the WHI, no effect of cHRT on stroke was seen in the first year of therapy. The risk ratio increased to 1.72 over the next 4 years and decreased to 0.66 in Year 6. Yearly confidence intervals have not been published, but, in the older WHI population, the overall absolute increased risk was 8 per 10 000 women per annum (0.08%). The final HR for stroke was 1.31 (adjusted 95% CI, 0.93–1.84).27 In the oestrogen-only arm of the WHI, the HR was 1.39 (adjusted 95% CI, 0.97–1.99).27 Again the prevalence of stroke is age-dependent and the numbers under age 60 years were small — too small to test the critical window hypothesis for stroke. An increased risk of transient ischaemic attacks and strokes must currently be presumed as likely in women initiating HRT many years after menopause.
Data from the WHI, observational studies and, recently, the Million Women Study (Level III-3) show a non-significant increase in ovarian cancer after 5 years of cHRT, but a significant increase after 5 years of unopposed oestrogen-only therapy.28,29 The increased absolute risk in the Million Women Study was estimated as one in 2500 users of HRT.29 This risk is mostly seen in women who have had a hysterectomy with ovarian conservation, and have taken oestrogen for more than 5 years. This group comprises about 8% of HRT users. Tibolone use was not associated with a rise in ovarian cancer.
In the cHRT arm of the WHI, a small decrease in these cancers was seen of around 8 per 10 000 women per annum.2 Oestrogen-only therapy had no effect on bowel cancer in the WHI.18
Symptom control and perceived improved quality of life are the main reasons for commencing HRT and for the high continuation rates. Systematic reviews (Level I) show that HRT very efficiently controls vasomotor symptoms and urogenital symptoms.30,31 In the WHI, women taking HRT had significantly reduced joint pains, and these increased on cessation of therapy. Disease-specific quality-of-life scores improve in women with menopausal symptoms who commence HRT, and are related to reductions in sleeplessness and tiredness and to increased libido.
The media scare after the initial WHI report in 2002 prompted medical review and cessation of long-term HRT in some users who had no further indication for its use. However, many more women inappropriately stopped HRT, or never started therapy, because of media and medical perception of its risks. Ironically, many women who experienced menopause after 2002 may have missed a therapeutic window for cardioprotection, and possible cognitive benefit, and also suffered unnecessary menopausal symptoms if they avoided, or their advisors denied them, the option of taking HRT. Many women have reported that their doctor or their pharmacist has said that HRT was too dangerous and they should use non-evidence-based complementary therapies.32
No complementary therapy has a greater effect than the placebo used in HRT trials.30,33 Some drugs acting on the brain (eg, selective serotonin reuptake inhibitors) are moderately better than placebo at reducing vasomotor symptoms.34 So-called “bioidentical” or “natural” unregistered hormones, individually compounded in untested doses and combinations, and often titrated using unvalidated salivary hormone assays, are mostly unassessed for efficacy or long-term safety.35 They should not be prescribed, and they exploit a regulatory loop hole that should be closed by the Therapeutic Goods Administration.
Although not a traditional HRT, tibolone is a steroid with oestrogenic, progestogenic and androgenic properties. It has been shown to have a good safety profile in short-term randomised controlled trials (Level II) with up to 4 years follow-up.36 It is an all-in-one, single dose, oral postmenopausal therapy, with a moderately effective action on menopausal and urogenital symptoms, libido and bone. Its potential lack of breast stimulation makes it a possible candidate for the treatment of menopausal symptoms in women with breast cancer. A 4-year placebo-controlled, randomised trial of tibolone in women with breast cancer (LIBERATE) will be reported later next year. There is a question mark about a small increased risk of stroke reported in one trial of older women with osteoporosis, but this result was confounded by unusually low numbers of strokes in the placebo group. The Million Women Study showed an association with breast cancer, which may have been confounded by selection of women with breast cancer for tibolone therapy. The LIBERATE study should help resolve this observation.
using lower HRT doses;
minimising or eliminating systemic progestogens (by use of intrauterine progestogen delivery systems);
using non-oral routes in some women; and
initiating HRT in symptomatic women from near menopause.
Systematic reviews of HRT show that the main two start-up side effects are irregular uterine bleeding (which is normal during the first few months of cHRT) and breast tenderness when excessive oestrogen is used.30 Longer-term therapy is appropriate for women with prolonged symptoms who are aware of the potential risks in their personal circumstances. When HRT is initiated near menopause for symptom control and subsequent improved quality of life, there are likely to be additional benefits (reduced fracture and cardiovascular risk, and possibly cognitive benefits) that outweigh the risks (which are not significantly raised in women under age 60 years). Beyond this age, women can try stopping HRT to see if their quality of life is maintained without therapy. However, some women have continuing symptoms into their seventh decade, and they should not be denied HRT if their therapy and risks are assessed on an individual basis, and each patient is aware of the risks.
1 National Health and Medical Research Council levels of evidence1
2 The main increased and decreased morbidities reported in the combined oestrogen and progestin arm and the oestrogen-only arm of the Women’s Health Initiative (WHI) trial, compared with placebo, per 10 000 women-years
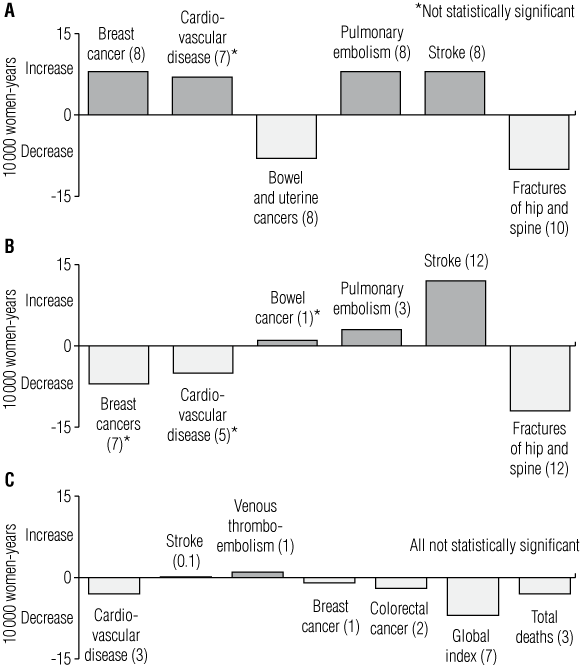
A: After 5 years of combined hormone replacement therapy (HRT).
B: After 7 years of oestrogen-only HRT.
C: Oestrogen-only HRT initiated at age 50–59 years.
- Alastair H MacLennan1
- University of Adelaide, Adelaide, SA.
I am Editor-in-Chief of Climacteric (Journal of the International Menopause Society). Through the University of Adelaide, I have received research funds and travel expenses from a variety of pharmaceutical companies including Acrux, Galen, Genentech, Ciba-Geigy, Organon, Rhone-Poulenc Rorer, Schering, Upjohn, and Wyeth.
- 1. National Health and Medical Research Council. A guide to the development, evaluation and implementation of clinical practice guidelines. Canberra: NHMRC, 1999. http://www.nhmrc.gov.au/publications/synopses/_files/cp30.pdf (accessed May 2007).
- 2. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomised controlled trial. JAMA 2002; 288: 321-333.
- 3. MacLennan AH, Taylor AW, Wilson DH. Hormone therapy use after the Women’s Health Initiative. Climacteric 2004; 7: 138-142.
- 4. Sturdee DW, MacLennan AH. Should epidemiology, the media and quangos determine clinical practice? Climacteric 2004; 7: 1-2.
- 5. Phillips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril 2005; 83: 558-566.
- 6. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006; 15: 35-44.
- 7. MacLennan AH, Sturdee DW. Long-term trials of HRT for cardioprotection — is this as good as it gets? Climacteric 2007; 10: 1-4.
- 8. Salpeter SR, Walsh JME, Greyber E, Salpeter EE. Coronary heart disease events associated with hormone therapy in younger and older women. J Gen Intern Med 2006; 21: 363-366.
- 9. Salpeter SR, Walsh ME, Greyber E, et al. Mortality associated with hormone therapy in younger and older women. J Gen Intern Med 2004; 19: 791-804.
- 10. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297: 1465-1477.
- 11. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997; 350: 1047-1059.
- 12. Greiser CM, Greiser EM, Doren M. Menopausal hormone therapy and risk of breast cancer. A meta-analysis of epidemiological studies and randomized controlled trials. Hum Reprod Update 2005; 11: 561-573.
- 13. Collins JA, Blake JM, Crosignani PG. Breast cancer risk with post menopausal hormonal treatment. Hum Reprod Update 2005; 11: 545-560.
- 14. Norman RJ, MacLennan AH. Current status of hormone therapy and breast cancer. Hum Reprod Update 2005; 11: 541-543.
- 15. Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003; 362: 419-427.
- 16. Singletary SE. Rating the risk factors for breast cancer. Ann Surg 2003; 237: 474-482.
- 17. Anderson GL, Chlebowski RT, Rossouw JE, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006; 55: 103-115.
- 18. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomised Controlled Trial. JAMA 2004; 291: 1701-1712.
- 19. Chen WY, Manson JA, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med 2006; 166: 1027-1032.
- 20. Cushman M, Kuller LH, Prentice R, et al, for the Women’s Health Initiative Investigators. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292: 1573-1580.
- 21. Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115: 840-842.
- 22. Sturdee DW, MacLennan AH. Prevention of osteoporosis is still a valid aim for hormone therapy. Climacteric 2005; 8: 97-98.
- 23. The North American Menopause Society. Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement. Menopause 2007; 14: 168-182.
- 24. MacLennan AH, Henderson VW, Paine BJ, et al. Hormone therapy, timing of initiation, and cognition in women older than 60 years: the REMEMBER pilot study. Menopause 2006; 13: 28-36.
- 25. Schumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. Women’s Health Initiative Memory Study. JAMA 2004; 291: 2947-2958.
- 26. Zandi PP, Carlson MC, Plassman BL, et al, for the Cache County Memory Study Investigators. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA 2002; 288: 2123-2129.
- 27. Clark JH. A critique of Women’s Health Initiative Studies (2002-2006). Nucl Recept Signal 2006; 4: e023.
- 28. La Vecchia C. Estrogen-progestogen replacement therapy and ovarian cancer: an update. Eur J Cancer Prev 2006; 15: 490-492.
- 29. Million Women Study Collaborators. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 2007; DOI:10.1016/S0140-6736(07)60534-0. (Published online Aril 19.)
- 30. MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004; (4): CD002978.
- 31. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; (4): CD001500.
- 32. MacLennan AH, Myers SP, Taylor AW. The continuing use of complementary and alternative medicine in South Australia: costs and beliefs in 2004. Med J Aust 2006; 184: 27-31.
- 33. Nedrow A, Miller J, Walker M, et al. Complementary and alternative therapies for the management of menopausal symptoms. A systematic evidence review. Arch Intern Med 2006; 166: 1453-1465.
- 34. Stearns V. Serotonergic agents as an alternative to hormonal therapy for the treatment of menopausal symptoms. Treat Endocrinol 2006; 5: 83-87.
- 35. MacLennan AH, Sturdee DW. The “bioidentical/bioequivalent” hormone scam. Climacteric 2006; 9: 1-3.
- 36. Kenemans P, Speroff L. Tibolone: clinical recommendations and practical guidelines. A report of the International Tibolone Consensus Group. Maturitas 2005; 51: 21-28.





Abstract
In 2002, when results of the Women's Health Initiative (WHI) randomised controlled trial of hormone replacement therapy (HRT) showed an increased occurrence of breast cancer and thromboembolism, up to two-thirds of women taking HRT stopped the therapy, often without medical consultation.
Recent analyses of the WHI data and other randomised controlled trials suggest that, although there are potential side effects and risks involved in taking HRT, these may be reduced by:
using lower HRT doses;
minimising or eliminating systemic progestogens;
using non-oral routes in some women; and
initiating HRT in symptomatic women near menopause.
When HRT is initiated near menopause for symptom control, there may be additional benefits (reduced fracture and cardiovascular risk) that outweigh the risks (which are not significantly raised in women under age 60 years).
Older women with continuing symptoms should not be denied HRT if their therapy and risks are assessed on an individual basis and each patient is aware of the risks.