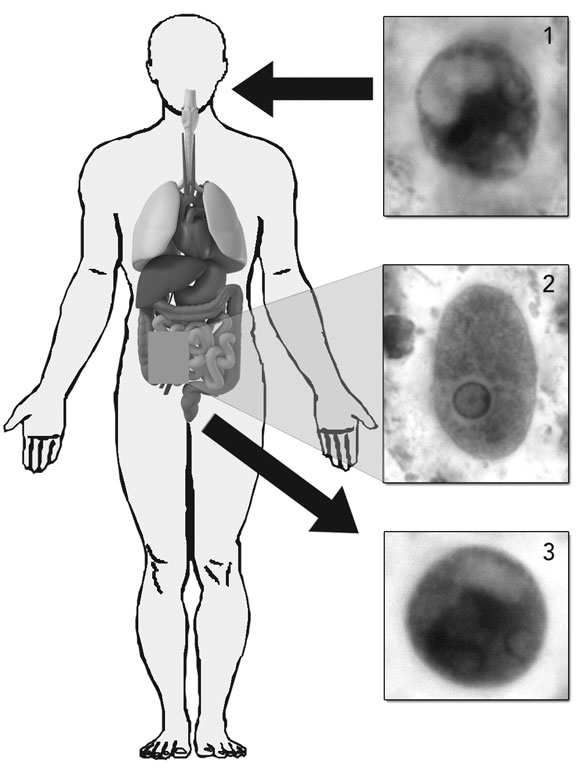
Amoebiasis, a disease caused by the intestinal protozoan parasite Entamoeba histolytica, is the third leading parasitic cause of death in humans after malaria and schistosomiasis. Globally, it is responsible for 40 000–100 000 deaths a year.1,2 E. histolytica has a worldwide distribution and is endemic in Australia, with locally acquired disease occurring in northern Australia (predominantly in Indigenous people) and, recently, in Sydney, among men who have sex with men (MSM).3-5 Other high-risk people in Australia include immigrants from countries of high endemicity (eg, India, South-East Asia) and travellers returning from such countries.1,6
The genus Entamoeba comprises six species that colonise the human intestinal lumen. E. histolytica (Box 1) was initially thought to be a single species, but isoenzyme and molecular studies have led to the reclassification of E. histolytica into two morphologically identical species: the pathogenic E. histolytica and the non-pathogenic E. dispar.1 E. moshkovskii, which is morphologically identical to E. histolytica and E. dispar but biochemically and genetically different, has been considered until recently to be primarily a free-living (non-pathogenic) amoeba. The early isolates of E. moshkovskii were free-living forms found in sewage, but human isolates have now been detected in North America, Italy, South Africa, Bangladesh, India, Iran and Australia.6 The pathogenic role of E. moshkovskii is yet to be adequately defined, but recent studies suggest that infection with this species can cause diarrhoea and other intestinal disorders.6 As E. moshkovskii is indistinguishable in its cyst and trophozoite forms from E. histolytica and E. dispar, it is not possible to differentiate the three species on the basis of traditional microscopic examination. Consequently, past studies on the prevalence of E. histolytica may be flawed if they did not consider the possible presence of E. dispar and E. moshkovskii.
Only one study has used adequate molecular techniques to determine the true prevalence of E. histolytica, E. dispar and E. moshkovskii in Australia. The study examined 5921 faecal samples submitted from patients with diarrhoea to a large metropolitan hospital in Sydney over a 4-year period. In 177 of the samples (3.0%), cysts and/or trophozoites microscopically resembling E. histolytica/dispar/moshkovskii were detected. Using molecular techniques, five patients were found to be infected with E. histolytica, 63 with E. dispar and 55 with E. moshkovskii. The study showed that, while E. dispar and E. moshkovskii are over 10 times more common, E. histolytica infections are nevertheless endemic in urban areas of Australia.7
Rates of asymptomatic carriage in immigrants to the United States are reported to be between 17% and 33%.8 In contrast, immigrants to Australia have a documented carriage rate of about 2%.9 In travellers returning to Australia, the carriage rate is unknown, and would vary greatly depending on the countries visited.8 International data reflect similar asymptomatic carriage rates (0.3%–10%), but differences between the countries visited and/or precautions taken need to be considered.6,10
A number of cases of amoebiasis have been reported from northern Australia in Indigenous and non-Indigenous patients from both remote and urban regions.3,11,12 While most cases predate the recognition of three separate species of Entamoeba, several recent cases have definitively identified E. histolytica as the cause of locally acquired invasive amoebiasis. The true prevalence of E. histolytica in northern Australian populations is unknown, as no studies using molecular techniques have been undertaken.
The prevalence of intestinal parasites in MSM is known to be higher than in heterosexual men. The possible explanation for this correlation is the practice of oral–anal sex.13 As E. histolytica is transmitted via the faecal–oral route, MSM are at increased risk of being infected. In Taiwan and Japan, E. histolytica has emerged as an important parasitic infection among MSM.14-17 This has also been documented in several Australian studies.5,6 Clinicians should be aware of this association, as MSM are at risk of invasive disease.
In most E. histolytica infections, symptoms are absent or very mild and represent “non-invasive” disease.1 Generally, asymptomatic patients never become symptomatic. They may excrete cysts for a short period of time, but the majority of these patients will clear the infection within 12 months. Patients with confirmed E. histolytica infection, even if they are asymptomatic, should be treated to eliminate the organism and prevent further transmission.
Only a small proportion of people infected with E. histolytica will go on to develop clinical disease, the most frequent manifestations being amoebic colitis and amoebic liver abscess.1 As amoebiasis is not a notifiable illness, the rate of invasive disease in Australia is unknown. Furthermore, as cases become more common (there were three cases at St Vincent’s Hospital, Sydney, in 2006), their “newsworthiness” diminishes, with the result that further reports on such cases are less likely to be published.
Patients with amoebic colitis present with a history of several weeks of abdominal pain and diarrhoea (usually characterised by blood, mucus and faecal leukocytosis). Fever occurs in less than 40% of patients. Toxic megacolon is a complication in about 0.5% of patients, and may be a consequence of inappropriate corticosteroid treatment.2 Steroids play an important role in the management of inflammatory bowel disease. As amoebic colitis is relatively uncommon in Australia, and as inflammatory bowel disease is part of the differential diagnosis of amoebic colitis, clinicians may overlook the less common diagnosis and treat patients inappropriately with corticosteroids. Progression to fulminant or necrotising colitis occurs in 0.5% of patients and is associated with a high mortality rate (40%) secondary to perforation, peritonitis or massive bleeding. Uncommon manifestations include amoebomas, rectovaginal fistulas and cutaneous involvement (especially after inappropriate steroid use) (Box 2).
Amoebic liver abscess is the most common extraintestinal manifestation of amoebiasis seen in Australia and has been reported in travellers and immigrants. Patients usually present within 5 months of contracting the disease. However, prolonged latency may occur.10,11 Clinical symptoms include fever (in 87%–100% of patients), malaise, and right upper quadrant pain with no concomitant colitis (in 60%–70% of patients). Biochemical parameters are usually abnormal but non-specific. Imaging studies (ultrasound, computed tomography and magnetic resonance imaging) have excellent sensitivity for detection of liver abscesses but are unable to distinguish amoebic liver abscesses from pyogenic abscesses or necrotic tumours. E. histolytica cysts and trophozoites are rarely found in the stools of patients with liver abscess, the majority of patients having no intestinal symptoms or history of dysentery. Therefore, the diagnosis depends on a high index of suspicion (eg, consistent travel history) and appropriate laboratory investigations. Complications associated with abscess rupture are dependent on which body cavity they rupture into. Such complications are rare, with the last documented case in Australia being in 1977.11 Mortality rates are low (< 1%) with appropriate treatment.
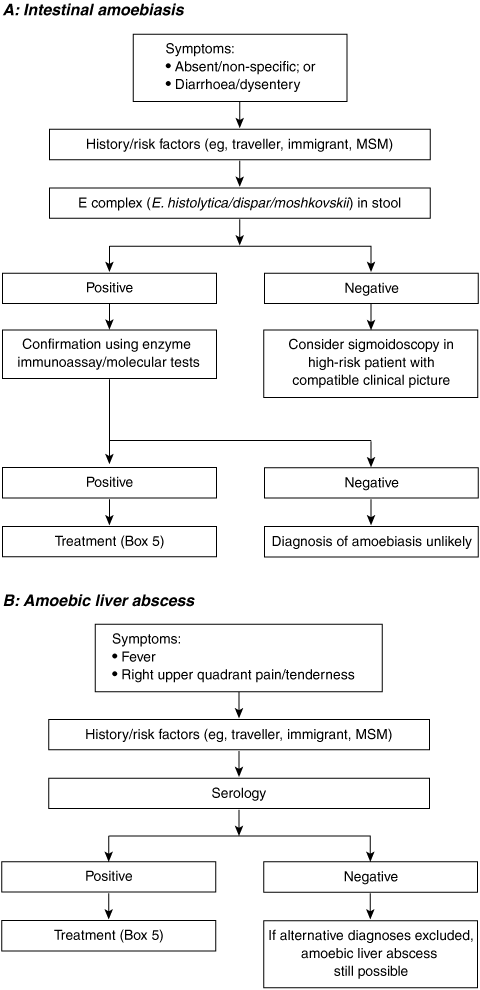
The diagnosis of amoebiasis is confirmed either by detecting E. histolytica parasites in the faeces or by detecting an antibody response to the parasite in the serum (Box 3, Box 4).
Unfortunately, many laboratories do not routinely perform permanent stains as they are time-consuming and laborious and require specific expertise. Multiple samples are required, as the organism is shed intermittently and the sensitivity of microscopy is poor.18 As E. histolytica is morphologically identical to the non-pathogenic species E. dispar and E. moshkovskii, microscopy can not distinguish between the three species, and further testing is required for speciation.19-21
Antigen detection methods use monoclonal antibodies directed against various proteins of E. histolytica. Some of these assays determine the presence of the E. histolytica/dispar/moshkovskii group, while others are specific for E. histolytica only. The two stool antigen detection kits commercially available in Australia — namely, the Entamoeba CELISA Path kit (Cellabs, Sydney, NSW) and the E. histolytica II kit (TechLab Inc, Blacksburg, Va, USA) — are able to distinguish E. histolytica from E. dispar and E. moshkovskii. For the more commonly used kit, sensitivities range from 80% to 99% and specificities from 86% to 98%.18,22-24 However, in a recent Australian study, the TechLab E. histolytica enzyme-linked immunosorbent assay (ELISA) test was found to cross-react with E. dispar and E. moshkovskii, thus limiting the utility of the test.6 The main advantage of these tests is that they are rapid (same day) and their interpretation is less subjective than microscopy.
Molecular methods using the polymerase chain reaction amplify E. histolytica genes from extracted faecal DNA. Sensitivity and specificity are high (80%–100% and 100%, respectively).22,25,26 The advantage of molecular detection is that it is extremely sensitive (able to detect < 1 parasite) and reliably able to differentiate non-pathogenic Entamoeba species from E. histolytica. Drawbacks of this method are the high level of expertise required and the cost. The availability of the test is limited, but at our institution it has proved extremely valuable.
Antibody tests (serology) are specific but have varying sensitivity, depending on the presence or absence of invasive disease and the type of invasive disease. The sensitivity of serology is about 95% for amoebic liver abscess and 84% for invasive intestinal disease.2 Antibody testing to diagnose carriage of E. histolytica is unhelpful, as the sensitivity is only 8%.2 A positive antibody test confirms the suspicion of invasive amoebic disease provided the patient has not had a disease episode in the recent past, as antibody titres can remain high for years after successful therapy.
Asymptomatic carriers of E. histolytica should be treated with a luminal agent to minimise the spread of disease and the risk of developing invasive disease.1
In patients with invasive disease, metronidazole should be used in conjunction with a luminal agent to eradicate the organism (Box 5).
The role of surgery is generally limited to patients with complications of invasive disease. Surgical drainage is generally unnecessary in amoebic liver abscess, as cure can be achieved with medical therapy alone.27,28 The role of radiologically guided percutaneous therapeutic aspiration in uncomplicated amoebic liver abscess is controversial,29 but it has been shown to be of some clinical benefit in patients with large abscesses. Aspiration should be considered in patients with an uncertain diagnosis, lack of response to medical therapy (persistent fever > 4 days), and large abscesses at risk of rupturing (especially left lobe abscesses, as these may rupture into the pericardial space).
2 Possible complications of Entamoeba histolytica infection
3 Diagnostic modalities
Novagnost Entamoeba histolytica IgG kit (IHA) (Dade Behring, Deerfield, Ill, USA) |
|||||||||||||||
5 Treatment of amoebiasis
Asymptomatic carriage (treat with luminal amoebicide ONLY)
Invasive disease (treat with tissue amoebicide and luminal amoebicide)
Liver abscess (treat with tissue amoebicide and luminal amoebicide)
-
Oral or intravenous metronidazole 750–800 mg three times daily for 14 days
-
Oral tinidazole 2 g once daily for 5 days and oral paromomycin* 500 mg three times daily for 7 days
- Sebastiaan J van Hal1
- Damien J Stark1
- Rashmi Fotedar1
- Debbie Marriott1
- John T Ellis2
- Jock L Harkness1
- 1 Department of Microbiology and Infectious Diseases, St Vincent’s Hospital, Sydney, NSW.
- 2 Department of Medical and Molecular Biosciences, University of Technology Sydney, Sydney, NSW.
None identified.
- 1. Stanley SL Jr. Amoebiasis. Lancet 2003; 361: 1025-1034.
- 2. World Health Organization. WHO/Pan American Health Organization/UNESCO report of a consultation of experts on amoebiasis. Wkly Epidemiol Rec WHO 1997; 72: 97-99.
- 3. McCarthy JS, Peacock D, Trown KP, et al. Endemic invasive amoebiasis in northern Australia. Med J Aust 2002; 177: 570. <MJA full text>
- 4. Weinke T, Friedrich-Jaenicke B, Hopp P, Janitschke K. Prevalence and clinical importance of Entamoeba histolytica in two high-risk groups: travelers returning from the tropics and male homosexuals. J Infect Dis 1990; 161: 1029-1031.
- 5. Stark DJ, Fotedar R, Ellis JT, Harkness JL. Locally acquired infection with Entamoeba histolytica in men who have sex with men in Australia. Med J Aust 2006; 185: 417. <MJA full text>
- 6. Stark D, Fotedar R, van Hal S. Prevalence of enteric protozoa in HIV-positive and HIV-negative men who have sex with men from Sydney, Australia. Am J Trop Med Hyg 2007; 76: 549-552.
- 7. Fotedar R, Stark D, Beebe N, et al. PCR detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii in stool samples from Sydney, Australia. J Clin Microbiol 2007; 45: 1035-1037.
- 8. US Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1993. MMWR Morb Mortal Wkly Rep 1994; 42: 1-73.
- 9. O’Brien DP, Leder K, Matchett E, et al. Illness in returned travelers and immigrants/refugees: the 6-year experience of two Australian infectious diseases units. J Travel Med 2006; 13: 145-152.
- 10. Shandera WX, Bollam P, Hashmey RH, et al. Hepatic amebiasis among patients in a public teaching hospital. South Med J 1998; 91: 829-837.
- 11. Zurauskas JP, McBride WJH. Case of amoebic liver abscess: prolonged latency or acquired in Australia? Intern Med J 2001; 31: 565-566.
- 12. Green PH. Amoebiasis: incidence at Royal North Shore Hospital, Sydney. Med J Aust 1977; 1: 11-13.
- 13. Law CL, Walker J, Qassim MH. Factors associated with the detection of Entamoeba histolytica in homosexual men. Int J STD AIDS 1991; 2: 346-350.
- 14. Ohnishi K, Kato Y, Imamura A, et al. Present characteristics of symptomatic Entamoeba histolytica infection in the big cities of Japan. Epidemiol Infect 2004; 132: 57-60.
- 15. Hung CC, Deng HY, Hsiao WH, et al. Invasive amebiasis as an emerging parasitic disease in patients with human immunodeficiency virus type 1 infection in Taiwan. Arch Intern Med 2005; 165: 409-415.
- 16. Nozaki T, Motta SR, Takeuchi T, et al. Pathogenic zymodemes of Entamoeba histolytica in Japanese male homosexual population. Trans R Soc Trop Med Hyg 1989; 83: 525.
- 17. Tsai JJ, Sun HY, Ke LY, et al. Higher seroprevalence of Entamoeba histolytica infection is associated with human immunodeficiency virus type 1 infection in Taiwan. Am J Trop Med Hyg 2006; 74: 1016-1019.
- 18. Haque R, Neville LM, Hahn P, et al. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol 1995; 33: 2558-2561.
- 19. Ali IK, Hossain MV, Roy S, et al. Entamoeba moshkovskii infections in children, Bangladesh. Emerg Infect Dis 2003; 9: 580-584.
- 20. Haque R, Ali IKM, Clark GC, et al. A case report of Entamoeba moshkovskii infection in a Bangladeshi child. Parasitol Int 1998; 47: 201-202.
- 21. Gonzalez-Ruiz A, Wright SG. Disparate amoebae. Lancet 1998; 351: 1672-1673.
- 22. Gonin P, Trudel L. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar isolates in clinical samples by PCR and enzyme-linked immunosorbent assay. J Clin Microbiol 2003; 41: 237-241.
- 23. Furrows SJ, Moody AH, Chiodini PL. Comparison of PCR and antigen detection methods for diagnosis of Entamoeba histolytica infection. J Clin Pathol 2004; 57: 1264-1266.
- 24. Solaymani-Mohammadi S, Rezaian M, Babaei Z, et al. Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol 2006; 44: 2258-2261.
- 25. Troll H, Marti H, Weiss N. Simple differential detection of Entamoeba histolytica and Entamoeba dispar in fresh stool specimens by sodium acetate-acetic acid-formalin concentration and PCR. J Clin Microbiol 1997; 35: 1701-1705.
- 26. Lebbad M, Svard SG. PCR differentiation of Entamoeba histolytica and Entamoeba dispar from patients with amoeba infection initially diagnosed by microscopy. Scand J Infect Dis 2005; 37: 680-685.
- 27. Lasserre R, Jaroonvesama N, Kurathong S, et al. Single-day drug treatment of amebic liver abscess. Am J Trop Med Hyg 1983; 32: 723-726.
- 28. Akgun Y, Tacyildiz IH, Celik Y. Amebic liver abscess: changing trends over 20 years. World J Surg 1999; 23: 102-106.
- 29. Weinke T, Grobusch MP, Guthoff W. Amebic liver abscess — rare need for percutaneous treatment modalities. Eur J Med Res 2002; 7: 25-29.





Abstract
Entamoeba histolytica is one of the most common parasitic infections worldwide, infecting about 50 million people and resulting in 40 000–100 000 deaths a year.
In Australia, people at risk of infection include immigrants, travellers returning from countries of high endemicity, Indigenous people, and men who have sex with men.
Clinical manifestations range from asymptomatic carriage to invasive disease. Amoebic colitis and amoebic liver abscess are the most common invasive manifestations observed in Australia.
Diagnosis depends on a high index of suspicion and laboratory investigations. Molecular methods (using the polymerase chain reaction) are the most sensitive for identifying and differentiating Entamoeba species.
Treatment should always include a luminal agent to eradicate colonisation, prevent spread and/or reduce the risk of invasive disease. Medical therapy can successfully cure invasive disease, including amoebic liver abscesses.