Clinical record
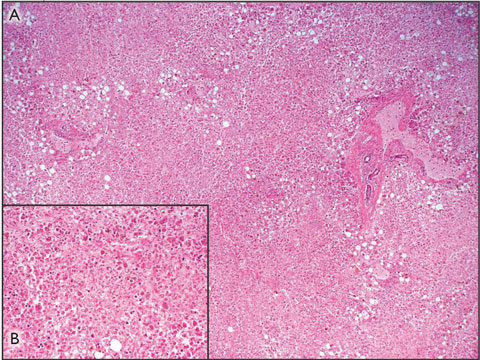
The patient was subsequently listed for liver transplantation, but died before a suitable donor became available. Postmortem examination confirmed that the cause of death was multisystem organ failure secondary to acute liver failure associated with paracetamol toxicity. The liver weighed 0.786 kg (normal weight in women, 1.2–1.4 kg), and the capsule appeared wrinkled. Histological assessment of the liver revealed a pattern of massive panlobular necrosis, with some mild fatty changes associated with preserved hepatocytes adjacent to portal tracts. The sinusoids contained increased numbers of inflammatory cells (Box 1).
Paracetamol toxicity is the most common cause of acute liver failure in Australia.3 A recent study of 662 patients from 22 tertiary care centres in the United States found that 48% of patients presenting with severe paracetamol hepatotoxicity had not intended to poison themselves, and took the drug for therapeutic purposes only. These patients tended to be older than patients who intentionally poison themselves.4 Paracetamol hepatotoxicity in those older than 40 years is associated with an increased risk of fulminant hepatic failure, death or liver transplantation.4,5 An analysis of 67 cases of “therapeutic misadventure” with paracetamol found that 60% of patients with accidental paracetamol poisoning had taken no more than 6 g paracetamol per day, and 40% had not exceeded the recommended maximum dose of 4 g per day.6 Risk factors for developing hepatic toxicity under these circumstances include prolonged fasting,7 regular excessive alcohol intake,6 or concurrent use of drugs that induce cytochrome P450 (CYP450), especially CYP450 2E1. Elderly patients with renal and cardiopulmonary insufficiency may also be at increased risk.8 Interestingly, there is little evidence that pre-existing liver disease increases the risk.1
Lessons from practice
Paracetamol poisoning at recommended therapeutic doses can occur. Accidental paracetamol poisoning should be suspected in any patient with acute liver failure.
Prolonged starvation can increase susceptibility to paracetamol poisoning by depleting hepatic glutathione stores.
Reduce the daily dose of paracetamol in people who are fasting, in heavy users of alcohol, or in those taking medications that induce cytochrome P450.
Paracetamol poisoning is typically associated with large rises in serum transaminase levels.
Patients with hepatic failure due to paracetamol poisoning should be referred early to a liver transplant unit for further management.
The link between fasting or CYP450 induction and increased susceptibility to paracetamol toxicity can be readily explained by reviewing the metabolism of this drug. When paracetamol is taken in standard doses in healthy individuals, more than 90% is conjugated to form inactive metabolites, which are then excreted in the urine. A small proportion of the paracetamol is metabolised by the CYP450 system to N-acetyl-p-benzoquinone imine (NAPQI), which, if allowed to accumulate, is toxic to the liver. Normally, NAPQI is conjugated with glutathione and the harmless products excreted in the urine (Box 2). Prolonged starvation can severely deplete the cosubstrates required for paracetamol conjugation and reduce glutathione stores, so that even therapeutic doses of the drug can result in the accumulation of toxic amounts of NAPQI (Box 2). Regular and prolonged treatment with paracetamol may be particularly dangerous in fasting patients, as ongoing metabolism of the drug leads to further consumption of already depleted glutathione stores. Alcohol and other drugs capable of inducing CYP450 predispose patients to paracetamol toxicity by increasing the production of NAPQI. In addition, chronic alcohol misuse leads to depletion of glutathione.
Between 1989 and 2004, 42 patients were referred to the Victorian Liver Transplant Unit with paracetamol-induced acute liver failure.9 In 11 of these (26%), the poisoning was clearly accidental. Eight patients recovered spontaneously, one survived after undergoing liver transplantation, and two died — including the patient described in this report. Thus, in Australia, severe life-threatening liver injury from accidental paracetamol poisoning appears to be quite uncommon. Nevertheless, this is a readily preventable syndrome of which both patients and the medical profession should be made more aware. In particular, clinicians should be cautious about prescribing regular doses of paracetamol for pain control in malnourished or fasting patients, and need to appropriately counsel patients who are regular users of the drug.
Although many cases of accidental paracetamol poisoning at therapeutic dosage have been described, controversy surrounds the actual incidence and mechanism. Unfortunately, as with our patient, it is impossible to determine whether larger quantities of paracetamol had been ingested either accidentally or intentionally.2,10
- 1. Benson GD. Acetaminophen in chronic liver disease. Clin Pharmacol Ther 1983; 33: 95-101.
- 2. Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction? Am J Ther 2000; 7: 99-114.
- 3. Gow PJ, Jones RM, Dobson JL, Angus PW. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J Gastroenterol Hepatol 2004; 19: 154-159.
- 4. Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364-1372.
- 5. Schmidt LE. Age and paracetamol self-poisoning. Gut 2005; 54: 686-690.
- 6. Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995; 22: 767-773.
- 7. Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994; 272: 1845-1850.
- 8. Bonkovsky HL, Kane RE, Jones DP, et al. Acute hepatic and renal toxicity from low doses of acetaminophen in the absence of alcohol abuse or malnutrition: evidence for increased susceptibility to drug toxicity due to cardiopulmonary and renal insufficiency. Hepatology 1994; 19: 1141-1148.
- 9. Lubel JS, Cheng RKY, Angus PW, Gow PJ. Fulminant hepatic failure due to accidental paracetamol overdose [abstract]. J Gastroenterol Hepatol 2004; 19: A254
- 10. Rumack BH. Acetaminophen misconceptions. Hepatology 2004; 40: 10-15.






None identified.