Bairnsdale or Buruli ulcer (BU) had been a rare disease in Victoria, but its incidence has risen markedly since 1990. Clinicians are increasingly called upon to diagnose and treat BU, but there is little information on the best approach. These guidelines reflect contemporary clinical practice in Victoria. They may not be applicable in countries with less developed health infrastructure. Existing guidelines, approved by the World Health Organization, are recommended for such areas.1
Mycobacterium ulcerans was discovered in 1948 by Australian scientists who were investigating a cluster of patients with unusual skin ulcers in the Bairnsdale region of eastern Victoria.2 M. ulcerans is related to the causative agents of tuberculosis and leprosy, but is transmitted from the environment rather than from person to person.3 The major virulence factor is a lipid toxin, mycolactone, which causes necrosis of fat and subcutaneous tissue.4
M. ulcerans infection is not fatal, but can result in significant morbidity and is expensive to treat.5,6 It has been reported in more than 30 countries, and is currently a significant public health problem in sub-Saharan Africa (Buruli ulcer).3 In Australia, there are active foci in coastal Victoria (Bairnsdale ulcer),7 Far North Queensland (Daintree ulcer),8 and near Rockhampton.9 Single cases also occur elsewhere in the Wet Tropics. The reason for this patchy distribution is unknown, but molecular typing has shown differences between strains isolated from patients in different regions.10
BU continues to occur in low numbers in the Bairnsdale/Gippsland Lakes area. In 1980, a new focus was noted near Tooradin and Warneet (Box 1) on Westernport,7 and this was followed by significant outbreaks at East Cowes on Phillip Island (1992–1995),7,11,12 Frankston and Langwarrin (1990–1998),7 St Leonards (1998–2001), Point Lonsdale (since 2002)13 and Barwon Heads and Ocean Grove (since 2005). There are also frequent single cases from other parts of the Mornington and Bellarine Peninsulas.
The incidence of BU in Victoria has increased sharply in recent years (Box 2). It is unlikely that this increase is due to improved diagnosis, as there has been considerable publicity since 1994, and all diagnostic polymerase chain reaction (PCR) assays have been available at a single institution since 1995.14 BU was made formally notifiable in Victoria from January 2004. Although the incidence in Victoria is currently only 1.4 per 100 000 overall, it is estimated that up to 6% of the permanent population of East Cowes (1992–1995)15 and 1% of the permanent population of Point Lonsdale have required treatment. Visitors to endemic areas are also at risk, and brief contact may be sufficient to become infected.
In patients presenting with unresolved cellulitis8 or a suspected necrotising spider bite, BU should be considered. At present, there is no evidence connecting spider bites to M. ulcerans.16
IS2404 PCR,8,14,17 which can be performed directly from ulcer swabs, approaches 100% sensitivity and specificity (P D R J, J A M F, independent personal observations). Culture is also diagnostic, but generally takes several weeks. A positive M. ulcerans PCR result is sufficient evidence to proceed to treatment.
Small lesions sometimes resolve spontaneously, but this is thought to be uncommon. The rate of progression varies among patients. Even though host immunity progressively develops during infection, ulcers may become very extensive. Relapse after treatment is not uncommon,18 so regular follow-up is recommended.
Cure with medical therapy alone is possible, and there is increasing interest in this approach in western Africa.1,19 However, in Victoria, where there is ready access to surgery, we believe that surgery or combined surgical and medical therapy is the most efficient way of effecting cure. The trend in management is towards conservative surgery with macroscopic removal of necrotic tissue and the use of adjuvant antibiotics. Patients may be best managed by a team, with surgeons working with infectious diseases specialists, GPs and allied health practitioners.
An orientated resection specimen that includes the skin and subcutaneous tissue should be submitted for histopathology. There is evidence from a recent case series that AFB or granulomatous inflammation or necrosis at the margins predicts relapse and the need for antibiotic treatment.20 If margins are clear, drug therapy is usually not necessary. However, clinicians are advised to discuss the risk of relapse versus antibiotic cost and risk of side effects to assist patients in making an informed choice. PCR testing of resection margins is not recommended.
Some clinicians favour the use of hyperbaric oxygen to assist healing, and there are supportive data from a mouse model.21 An Italian group is investigating the use of adjuvant hyperbaric oxygen for BU in Benin, but results are not yet available.
In the laboratory, M. ulcerans is susceptible to a range of antibiotics. The WHO recommends the combination of oral rifampicin and parenteral streptomycin for initial treatment.1 The use of streptomycin (replaced by amikacin in Australia) combined with oral rifampicin is supported by animal data,22 a published case series from western Africa,19 and observational data presented at the annual meetings of the WHO Global Buruli Ulcer Initiative.23 In Victoria, where many patients are elderly, clinicians have encountered problems with toxicity from amikacin (renal, balance or hearing difficulties), and may prefer less toxic oral combinations. However, there is less human evidence to support this practice, and in animal models, oral-only combinations are less effective at killing M. ulcerans,24 with the possible exception of rifampicin plus moxifloxacin.25
Antibiotic treatment is relatively expensive, may require monitoring for toxicity, and is generally given for at least 3 months in total, with the intravenous component typically for 4 weeks. However, appropriate use of antibiotics allows more conservative surgery and reduces the risk of relapse. Box 3 presents recommendations for use of oral antibiotics, and Box 4 contains recommendations for use of intravenous amikacin.
The use of antibiotics for the treatment of M. ulcerans is “off label”. The usual precautions should be taken whenever new drugs are prescribed. Always refer to the full product information. Ciprofloxacin is not generally recommended in prepubertal children, as studies in animal models have demonstrated arthropathy.26 However, there is limited evidence from human studies that short courses of ciprofloxacin may be safe in children.27 Patients should be warned about the small risk of drug-related hepatitis associated with combinations that include rifampicin, and liver function tests should be monitored periodically. There is a small risk of tendinitis associated with quinolone use, and an alternative agent should be found if tendon stiffness develops during treatment.
It is not understood why M. ulcerans outbreaks occur in new areas or why the disease has spread westwards from the original endemic area near the Gippsland Lakes. There are no public health interventions that can remove M. ulcerans from the environment, although there may be a natural cycle of human infection that lasts several years, after which the incidence of new cases in a given area abates. There is circumstantial evidence that nutrient enrichment of very low-lying coastal environments may be a factor in the appearance of outbreaks.7,12,28
M. ulcerans has been detected by PCR in plant material and mud obtained from swamps in endemic areas,29 a golf course irrigation system that used recycled water,28,29 and from aquatic water insects in Africa.30 At Point Lonsdale, M. ulcerans has been detected by PCR in soil and leaf litter from a stormwater drainage system, mud from a lake, and about 0.5% of more than 10 000 mosquitoes trapped during 2004–2006 (unpublished data). It is yet to be determined whether mosquitoes play a role in human disease.
Studies from African endemic areas have reported that farming activities close to rivers31 and swimming32 may be risk factors, and that wearing trousers31 and a shirt33 when working outdoors appears protective. A recent case–control study performed on the Bellarine Peninsula has shown that direct exposure to the environment and to mosquitoes are risk factors, and wearing protective clothing and insect repellent appears protective (unpublished data).
BU is notifiable in Victoria and up-to-date figures are displayed on a publicly accessible website.34 However, it is recommended that the exact location of cases be documented (eg, by postcode), and that clinical photographs and notes on diagnosis be made available through the site.
2 Notified cases of Mycobacterium ulcerans infection in Victoria since 1998
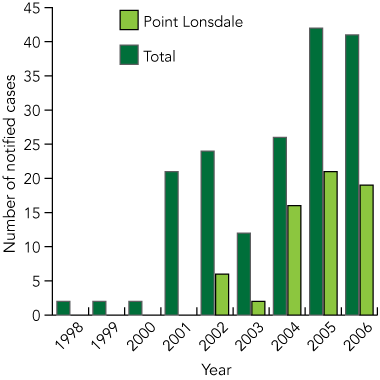
Notification was voluntary before 2004. Data for 2006 are only to August. Data are from the Victorian Department of Human Services and reference 7.
3 Recommendations for oral antibiotic use in the treatment of Bairnsdale ulcer
Combination antibiotics are recommended
For a total of 3 months when the histology of resection margins shows either necrosis or acid fast bacilli or granulomata
or when the initial lesion was large enough to require grafting
or for complex, recurrent disease or where surgical resection is necessarily incomplete. Consider including intravenous amikacin in this situation (see Box 4)
Rifampicin 10 mg/kg per day up to 600 mg daily for 3 months, plus
clarithromycin 500 mg twice daily for 3 months
or ciprofloxacin 500–750 mg twice daily for 3 months
or moxifloxacin 400 mg once daily for 3 months (not recommended for children)
Rifampicin 10–20 mg/kg per day in one daily dose; not to exceed 600 mg per day
Clarithromycin 15–30 mg/kg per day in two divided doses for children < 12 years; dose as for adults when > 12 years, not to exceed adult doses
Ciprofloxacin 20 mg/kg per day in two divided doses, not to exceed adult doses
4 Recommendations for use of intravenous amikacin in the treatment of Bairnsdale ulcer
When to use intravenous amikacin (with oral rifampicin)
Severe or extensive disease
When deep structures (such as tendons, nerves, joint capsules, major blood vessels) that need to be preserved are involved
Large lesions that could not be fully resected
Major relapses
Osteomyelitis
When trying to avoid or minimise surgery (eg, lesions involving the eye or face)
Initial therapy of acute oedematous disease
How to administer intravenous amikacin (in adults)
Amikacin 15 mg/kg (ideal weight) intravenously (maximum 1000 mg) daily on 5–7 days each week for 4–8 weeks
Monitor renal function
Monitor hearing and vestibular function at least monthly
Monitor trough levels once or twice weekly
Aim for trough level < 1 mg/L. Dosing should be spaced to three times weekly if drug accumulation is detected (ie, trough levels begin to rise) or if prolonged continuation therapy is envisaged
Appendix: Conference delegates
- Paul D R Johnson1
- John A Hayman2
- Tricia Y Quek3,4
- Janet A M Fyfe5
- Grant A Jenkin6
- John A Buntine7
- Eugene Athan3,4
- Mike Birrell8
- Justin Graham6
- Caroline J Lavender5
- on behalf of the Mycobacterium ulcerans Study Team*
- 1 Department of Infectious Diseases, Austin Health, Melbourne, VIC.
- 2 Department of Anatomy and Cell Biology, Monash University, Melbourne, VIC.
- 3 Department of Clinical and Biomedical Sciences, University of Melbourne, Melbourne, VIC.
- 4 Department of Infectious Diseases, Barwon Health, Geelong, VIC.
- 5 Victorian Infectious Diseases Reference Laboratory, Melbourne, VIC.
- 6 Southern Health, Melbourne, VIC.
- 7 Cornell Specialist Centre, Melbourne, VIC.
- 8 Point Lonsdale Medical Group, Point Lonsdale, VIC.
We thank Mr Dallas Wilson for his expert assistance with audiovisuals and recording of the conference proceedings.
None identified.
- 1. World Health Organization. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). http://www.who.int/buruli/information/antibiotics/en/index.html (accessed Jun 2006).
- 2. MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol 1948; 60: 93-122.
- 3. Johnson PDR, Stinear T, Small PLC, et al. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Med 2005; 2(4): e108.
- 4. George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 1999; 283: 854-857.
- 5. Asiedu K, Etuaful SA. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg 1998; 59: 1015-1022.
- 6. Drummond C, Butler JR. Mycobacterium ulcerans treatment costs, Australia. Emerg Infect Dis 2004; 10: 1038-1043.
- 7. Johnson PDR, Veitch MGK, Leslie DE, et al. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust 1996; 164: 76-78.
- 8. Jenkin GA, Smith M, Fairley M, Johnson PDR. Acute, oedematous Mycobacterium ulcerans infection in a farmer from far north Queensland. Med J Aust 2002; 176: 180-181. <MJA full text>
- 9. Francis G, Whitby M, Woods M. Mycobacterium ulcerans infection: a rediscovered focus in the Capricorn Coast region of central Queensland [letter]. Med J Aust 2006; 185: 179-180. <MJA full text>
- 10. Stinear TP, Jenkin GA, Johnson PD, Davies JK. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol 2000; 182: 6322-6330.
- 11. Flood P, Street A, O’Brien P, Hayman J. Mycobacterium ulcerans infection on Phillip Island, Victoria. Med J Aust 1994; 160: 160.
- 12. Johnson PD, Veitch MG, Flood PE, Hayman JA. Mycobacterium ulcerans infection on Phillip Island, Victoria. Med J Aust 1995; 162: 221-222.
- 13. Tiong A. The epidemiology of a cluster of Mycobacterium ulcerans infections in Point Lonsdale. Victorian Infect Dis Bull 2005; 8: 2-4. http://www.health.vic.gov.au/ideas/downloads/bulletin/vidbv8i1.pdf (accessed Aug 2006).
- 14. Ross BC, Marino L, Oppedisano F, et al. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 1997; 35: 1696-1700.
- 15. Veitch MGK, Johnson PDR, Flood PE, et al. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol Infect 1997; 119: 313-318.
- 16. Atkinson RK, Farrell DJ, Leis AP. Evidence against the involvement of Mycobacterium ulcerans in most cases of necrotic arachnidism. Pathology 1995; 27: 53-57.
- 17. Russell FM, Starr M, Hayman J, et al. Mycobacterium ulcerans infection diagnosed by polymerase chain reaction. J Paediatr Child Health 2002; 38: 311-313.
- 18. Debacker M, Aguiar J, Steunou C, et al. Buruli ulcer recurrence, Benin. Emerg Infect Dis 2005; 11: 584-589.
- 19. Etuaful S, Carbonnelle B, Grosset J, et al. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother 2005; 49: 3182-3186.
- 20. O’Brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australian case series. Med J Aust 2007; 186: 58-68. <MJA full text>
- 21. Krieg RE, Wolcott JH, Confer A. Treatment of Mycobacterium ulcerans infection by hyperbaric oxygenation. Aviat Space Environ Med 1975; 46: 1241-1245.
- 22. Dega H, Bentoucha A, Robert J, et al. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother 2002; 46: 3193-3196.
- 23. World Health Organization. Global Buruli ulcer initiative. http://www.who.int/buruli/en/ (accessed Aug 2006).
- 24. Bentoucha A, Robert J, Dega H, et al. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother 2001; 45: 3109-3112.
- 25. Ji B, Lefrancois S, Robert J, et al. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother 2006; 50: 1921-1926.
- 26. Yoshida K, Yabe K, Nishida S, et al. Pharmacokinetic disposition and arthropathic potential of oral ofloxacin in dogs. J Vet Pharmacol Ther 1998; 21: 128-132.
- 27. Zimbabwe Bangladesh South Africa (Zimbasa) Dysentery Study Group. Multicenter, randomized, double blind clinical trial of short course versus standard course oral ciprofloxacin for Shigella dysenteriae type 1 dysentery in children. Pediatr Infect Dis J 2002; 21: 1136-1141.
- 28. Ross BC, Johnson PDR, Oppedisano F, et al. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol 1997; 63: 4135-4138.
- 29. Stinear TP, Davies JK, Jenkin GA, et al. Identification of Mycobacterium ulcerans in the environment from regions in southeast Australia in which it is endemic with sequence-capture PCR. Appl Environ Microbiol 2000; 66: 3206-3213.
- 30. Marsollier L, Robert R, Aubry J, et al. Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol 2002; 68: 4623-4628.
- 31. Marston BJ, Diallo MO, Horsburgh CR Jr, et al. Emergence of Buruli ulcer disease in the Daloa region of Cote d’Ivoire. Am J Trop Med Hyg 1995; 52: 219-224.
- 32. Aiga H, Amano T, Cairncross S, et al. Assessing water-related risk factors for Buruli ulcer: a case–control study in Ghana. Am J Trop Med Hyg 2004; 71: 387-392.
- 33. Raghunathan PL, Whitney EA, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): results from a case–control study in Ghana. Clin Infect Dis 2005; 40: 1445-1453.
- 34. Victorian Department of Human Services. Notifications of infectious diseases: summary reports. http://www.health.vic.gov.au/ideas/downloads/daily_reports/rptVictorianSummary.pdf (accessed Aug 2006).





Abstract
Mycobacterium ulcerans causes slowly progressive, destructive skin and soft tissue infections, known as Bairnsdale or Buruli ulcer (BU).
Forty-six delegates with experience in the management of BU attended a 1-day conference in Melbourne on 10 February 2006, with the aim of developing a consensus approach to the diagnosis, treatment and control of BU. An initial draft document was extended and improved during a facilitated round table discussion.
BU is an environmental infection that occurs in specific locations. The main risk factor for infection is contact with an endemic area.
Prompt cleaning of abrasions sustained outdoors, wearing protective clothing, and avoiding mosquito bites may reduce an individual’s risk of infection.
BU can be rapidly and accurately diagnosed by polymerase chain reaction testing of ulcer swabs or biopsies.
Best outcomes are obtained when the diagnosis is made early. To aid early diagnosis, health authorities should keep local populations informed of new outbreaks.
BU is best treated with surgical excision, which, if possible, should include a small rim of healthy tissue. For small lesions this may be all that is required. However, there is a role for antibiotics for more extensive disease, and their use may allow more conservative surgery.