The main difference between the upper and lower airways is that upper airway patency is largely influenced by vascular tone, whereas, in the lower airway, airflow is influenced predominantly by smooth muscle function. Direct tests of bronchial hyper-responsiveness (methacholine and histamine challenges) result in smooth muscle constriction and measurable airflow obstruction. In the nose, both agents cause multiple symptoms, including sneezing, anterior rhinorrhoea and nasal obstruction, the latter due to effects on vasculature. However, as there is considerable overlap in the measurable nasal obstruction between people with or without rhinitis, nasal challenges with these agents have little clinical usefulness. Hence it is much easier to demonstrate airway hyper-responsiveness (AHR) in the bronchi than in the nose. The different anatomy also explains the differential effects of drugs such as antihistamines and β2-agonists on the upper and lower airways.1,2
Inspired air is conditioned by the nose so that, by the time it reaches the trachea, the air temperature is about 32°C and the humidity about 98%. Nitric oxide produced by the sinuses (eNO) has a bronchodilatory activity that has been postulated to partially protect the patency of the lower airway.1
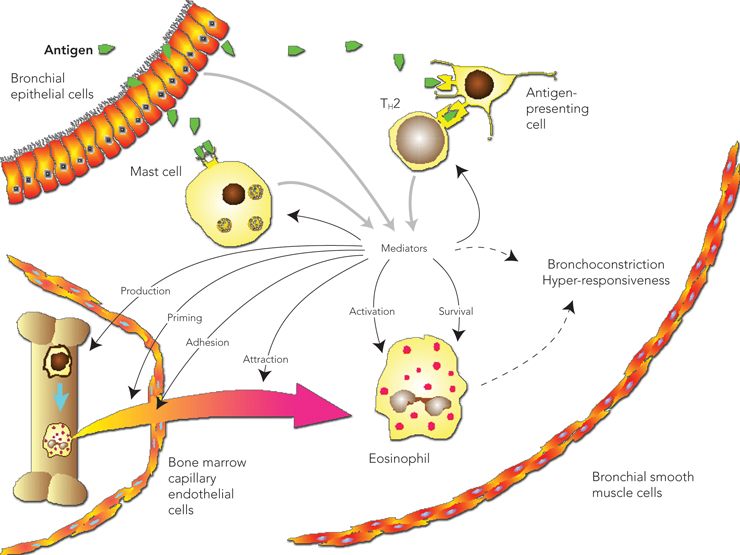
Allergic disease is the result of an immune response to external antigens, which leads to production of antibodies that are typically, but not exclusively, IgE antibodies. Allergic airway disease results from hypersensitivity or IgE-mediated reactions when inhaled allergen reacts with cells bearing IgE antibodies (typically mast cells and basophils).3 Cross-linking of allergen-specific IgE molecules bound to cells by allergen particles results in the release of granule-associated mediators (eg, histamine, tryptase), membrane lipid-derived mediators (eg, leukotrienes) and cytokines. The initial reaction, the early allergic response (maximal at 10–20 minutes), is usually followed by a late allergic response (within 2–6 hours) in both the upper and lower airways. The later response is associated with an eosinophilic and T cell (CD4+) tissue infiltrate.
The symptoms of rhinitis are rhinorrhoea, nasal obstruction, itch and sneeze, while symptoms of asthma are sputum, wheeze, chest tightness and cough. Until recently, allergic rhinitis has been classified as either seasonal or perennial, but, as there is an overlap between these categories in many patients, a new classification of intermittent versus persistent rhinitis has been proposed (Box 1).4 Asthma is currently defined by both histological and functional parameters, with further descriptors relating to disease severity (Box 1).5
The UAD hypothesis proposes that any disease process that affects the upper airway is likely to affect the lower airway, and vice versa, by both direct and indirect means. It is postulated that rhinitis and asthma represent the manifestations of one syndrome in two parts of the respiratory tract, the upper and lower airways, respectively. At the low end of the severity spectrum, rhinitis may occur alone; in the middle range of the spectrum, rhinitis and AHR may be present; and, at the high end, rhinitis and asthma may both be present, with the severity of each condition tracking in parallel.6 Disease manifestations in the upper and lower airways may be linked via a systemic inflammatory response. The hypothesis may hold for not only allergic but also non-allergic presentations.
Acute and chronic changes in the upper and lower airways in response to an allergic stimulus are summarised in Box 2. Although the nose is usually the first site of exposure to allergens or other noxious substances, the presence of nasal epithelial damage is minimal, whereas, in the bronchi, marked epithelial disruption may be present. Thus, it is postulated that the nasal mucosa has developed protective mechanisms that minimise remodelling and enhance epithelial regeneration.7 In support of this hypothesis, animal models of airway inflammation show that, although the majority of allergen is deposited in the nose, more inflammation occurs in the lower airway.2 Basement membrane thickening, a consistent hallmark of lower airway remodelling in asthma, is present even in children with asthma, and also in atopic patients without asthma and patients with allergic rhinitis; however, it has not been reproducibly demonstrated to occur in the nose.
Inflammatory changes can be detected in both the upper and lower airways without accompanying clinical symptoms. In patients with rhinitis, lower airway inflammation can be demonstrated by increased levels of eNO and by eosinophils found in induced sputum, bronchoalveolar lavage fluid and bronchial biopsies. Similarly, in patients with asthma, nasal biopsies show eosinophilic inflammation, even in those who do not have symptoms of rhinitis.8 After direct allergen challenge in one part of the airway, an inflammatory response can be shown in both the target area and the reciprocal part of the airway within 24–48 hours of the challenge.9,10 These two seminal studies have underpinned further development of the UAD hypothesis.
Several mechanisms to explain the interaction between the upper and lower airways have been proposed (Box 3). The most likely mechanism is that localised airway inflammation leads to a systemic response, with bone marrow involvement resulting in the release of progenitor cells that are then recruited to tissue sites (Box 4).11
Are there possible exceptions to the UAD hypothesis, and is it purely an allergic phenomenon?
Several observations support the concept of UAD existing outside a purely allergic context. For example, infective inflammation occurring in both the upper and lower airways is often attributed to a direct viral effect at both sites, but the two may also be linked by indirect means (Box 3).1
Intrinsic asthma, such as aspirin-exacerbated respiratory disease (Samter’s triad of aspirin sensitivity, nasal polyposis and asthma), demonstrates that upper and lower airway inflammation can sometimes be found in the absence of atopy (Box 5).1 A study by Gaga et al8 assessed nasal biopsies in 19 non-atopic subjects with asthma, with or without nasal symptoms. Both study groups showed similarly high nasal eosinophil counts compared with normal patients. Given that lower airway inflammation is accepted as universal in asthma (Box 1), this finding supports the coexistence of upper and lower airway inflammation in non-atopic subjects. However, a larger number of subjects would need to be studied to establish whether this finding can be generalised.
The presence of lower AHR without symptoms of clinical asthma has been well documented in population studies. In addition, the relationship between AHR and inflammation is complex, with many studies not supporting a relationship between the two. As airway inflammation is central to the concept of UAD, it is possible that the upper airway may not be involved in isolated, non-asthmatic AHR. Eosinophilic bronchitis, a newly identified condition of the lower airway associated with persistent cough, is an example of lower airway inflammation without AHR, but, as yet, upper airway disease has not been looked for in association with this condition.15
Another lower airway inflammatory disease, chronic obstructive pulmonary disease (COPD), may also be an example of UAD. COPD is associated with elevated levels of intranasal interleukin-8 (IL-8), which correlates with sputum levels, indicating concomitant upper and lower airway inflammation.16 In COPD exacerbation there is, as well as a pan-airway response, evidence of a systemic response as determined by elevated serum levels of IL-6 and C-reactive protein. But again, with a predominantly infective stimulus, it is more difficult to separate site-specific effects of the provoking agent from underlying systemic processes.17
The prevalence of atopy, rhinitis and asthma have all increased worldwide over recent decades.18 In a series of population studies of Australian schoolchildren in Belmont, New South Wales, hay-fever prevalence increased between 1982 and 2002 from 9.1% to 38.4%, and prevalence of current asthma (defined as recent wheeze and AHR) increased from 4.5% to 11.3%.19 Other studies indicate that rhinitis is a risk factor for later developing asthma (odds ratio, 2.5919 to 11.020).
An analysis of the same Belmont population showed that the presence of hayfever at the age of 8–10 years was predictive of the presence of troublesome asthma by the age of 23–25 years (likelihood ratio, 2.14; 95% CI, 1.59–2.89).21
It is well documented that allergic rhinitis is associated with increased AHR, even if there is no diagnosis of asthma. People subject to allergic rhinitis have demonstrable AHR both outside the pollen season (11%–73%) and during the pollen season (50%).1 Clinical studies indicate that 80%–100% of patients with asthma have rhinitis and 50% of patients with rhinitis have asthma, and that both the presence and severity of rhinitis are associated with worse asthma outcomes.1
An association between asthma and sinusitis has long been recognised. In a recent study, 100% of subjects with severe asthma (requiring steroid treatment) had abnormal sinus computed tomo-graphy scans versus 77% of subjects with mild to moderate asthma.22 However, perhaps the most direct evidence of the relationship between sinusitis and asthma is provided by studies that show significant improvement in asthma symptoms when sinusitis is appropriately treated.23
The effectiveness of different therapies for managing rhinitis and asthma is summarised in Box 6. Here, we expand only on the therapies that can help to further explore the relationship between the upper and lower airways and potentially unite management of conditions affecting either or both. We also explore the role of allergen avoidance.
A Cochrane database meta-analysis suggests that intranasal corticosteroids are generally effective in controlling asthma, but studies refuting this finding do exist.24 The effect of inhaled corticosteroids on allergic rhinitis has rarely been studied, but Grieff et al demonstrated reduced nasal and blood eosinophilia and reduced seasonal nasal symptoms with the use of inhaled budesonide.25 Stelmach et al, in a double-blind, parallel, three-group study comparing the use of intranasal budesonide (400 μg), inhaled budesonide (1000 μg), or both, showed that intranasal therapy alone improved asthma symptoms (although it did not increase lung function), and that this effect was as good as the inhaled therapy or the combination of inhaled and intranasal therapy.26 Adams et al,27 in a retrospective study of 13 844 patients with asthma, showed that intranasal corticosteroid therapy reduced the risk of emergency department visits (overall relative risk, 0.70; 95% CI, 0.59–0.94), independently of whether or not patients used inhaled corticosteroids.
Antihistamines. Oral antihistamines are generally believed to have little effect on asthma. However, when used to treat seasonal allergic rhinitis, they have been shown to reduce the symptoms of asthma during the pollen season, although they had no effect on lung function.28 In the Early Treatment of the Atopic Child study,29 children aged 12–24 months with atopic dermatitis and a high risk of asthma (because of their parental history) were given cetirizine or placebo for 18 months and followed up for a further 6 months. Infants with evidence of sensitivity to house dust mite, grass pollen or both who were treated with cetirizine were significantly less likely to have developed asthma (50% reduction) compared with those treated with placebo over the 18 months. This study is being followed by the Early Prevention of Asthma in Atopic Children study, but results are not yet published. At present, the prophylactic use of cetirizine to prevent asthma at at-risk children cannot be recommended. There is no study examining the effect of intranasal antihistamines on asthma.
Newer systemic therapies. Newer systemic therapies include the use of oral leukotriene receptor antagonists (eg, montelukast) and subcutaneous anti-IgE monoclonal antibody therapy (omalizumab). These therapies target specific parts of the inflammatory process and provide evidence for the concept that leukotriene mediators and the downstream effects of IgE–allergen interactions play a part in the pathophysiology of the allergic immune response. It is likely that a range of other, similar therapies will be developed, but these are not always predictably successful, as has been illustrated by the lack of effect of anti-IL-5 in asthma trials.30
Leukotriene receptor antagonists cause bronchodilation and can block the acute effects of allergen exposure in both the upper and lower respiratory tract, as well as controlling chronic airway disease.31,32 Although not as effective as topical corticosteroids for controlling asthma (montelukast 10 mg/day is said to be equivalent to 400 μg/day of inhaled steroids),32 leukotriene receptor antagonists have an important role to play in childhood asthma, mainly because of their better safety profile. If patients have concurrent symptoms of rhinitis and asthma, there is the potential advantage of using a single oral therapy for both diseases.
Omalizumab is used to treat allergic disease, showing benefit in patients with moderate to severe allergic asthma and in those with seasonal and perennial allergic rhinitis.33 Omalizumab reduces serum levels of IgE and down-regulates the number of IgE receptors. It also reduces the number of tissue eosinophils (both nasal and bronchial), bronchial IgE positive cells (mast cells), T cells and B cells. Moreover, it is of benefit to patients who have poorly controlled asthma despite using high-dose inhaled corticosteroids. Indeed, patients who are the best “responders” to anti-IgE treatment are those with asthma at the more severe end of the spectrum.
Systemic anti-IgE therapy with omalizumab has been shown to improve symptoms, quality of life and disease control (asthma exacerbations) in patients with concomitant asthma and persistent allergic rhinitis.33 However, as it is an expensive drug with consequent limited availability, it does not have a significant role in treating asthma and rhinitis at the present time.34
Allergen immunotherapy delivered by subcutaneous injection has been used successfully to treat both allergic rhinitis and asthma (Box 7).35,36 A meta-analysis of allergen immunotherapy that included 75 prospective, randomised controlled trials of immunotherapy for asthma showed a reduction in the need for medication, a reduction in bronchial hyper-responsiveness, and improvement in forced expiratory volume in 1 second (FEV1).36 Studies in children have shown that immunotherapy reduces the likelihood of developing asthma37,38 and the risk of developing new allergies.37
In a European study, 205 children aged 6–14 years with seasonal rhinoconjunctivitis were treated for 3 years with allergen immunotherapy.38 Before the start of the study, all subjects had moderate to severe hayfever symptoms and 20% had mild seasonal asthma. Active therapy resulted in a statistically significant reduction in rhinitis, conjunctivitis and bronchial reactivity compared with the control group. Further, active therapy led to a 50% reduction in the development of newly diagnosed asthma.
Fact or fiction — true or false?
1. Patients with united airway disease always have both asthma and rhinitis.
2. United airway disease only applies to patients with allergy.
3. Treating allergic rhinitis will reduce the symptoms of asthma.
4. All patients with asthma are allergic to some allergen.
3. True. This has been demonstrated in a recent double-blind trial.26
Allergen immunotherapy can also prevent the development of sensitisation to new allergens. A group of 134 children who had intermittent asthma (with or without rhinitis) and who were singly sensitised to dust mite were enrolled into active treatment and placebo groups. Specific dust mite immunotherapy was given for 3 years and subjects were followed up for a further 3 years. Only 24.6% of children in the immunotherapy group developed new sensitisation (most commonly to pollens), compared with 66.7% in the control group (P < 0.001).37
Sublingual/oral immunotherapy (SLIT), which is easier to administer to children than injection therapy and also has the advantage of carrying minimal or no risk of anaphylaxis, leads to improvement in symptoms of rhinitis. There is limited evidence that SLIT may prevent the development of allergic disease in childhood.39
The value of allergen avoidance as a therapeutic measure for rhinitis or asthma, or both, has long been controversial. Avoidance studies can be categorised according to the specific allergen avoided and the outcome measures: relief of current symptoms or the primary prevention of allergic disease. In the case of occupational asthma, allergen avoidance is highly recommended and often successful.3
Although a meta-analysis of all avoidance studies of ubiquitous allergens such as dust mite allergen provided little evidence of benefit,40 some of the studies included in the analysis did not show evidence of reduced allergen exposure. Allergen avoidance directed at a particular allergen to which the patient is allergic (eg, cat allergen) may be more effective, but the data are conflicting.41 Early childhood interventional studies in which there have been measurable reductions in allergen levels (eg, in dust mite exposure) have provided little evidence of disease prevention.42
These results are perplexing and have led to the realisation that the relationship between the level of allergen exposure and the degree of sensitisation is complex, with immunological tolerance occurring in some cases (eg, to cat allergen). However, the increase in prevalence of allergic diseases, particularly in affluent societies, implicates environmental factors that need to be identified before adequate preventive measures can be undertaken. Current recommendations for allergen avoidance for patients are summarised at <http://www.allergy.org.au>.
Mild: normal sleep and no impairment of daily activities, sport, leisure, work or school; no troublesome symptoms.
Moderate to severe: one or more of (a) abnormal sleep; (b) impairment of daily activities, sport or leisure; (c) problems caused at work or school; and (d) troublesome symptoms.
Mild: occasional symptoms, but no nocturnal or early morning symptoms; use of short-acting β-agonists less than twice a week; no previous hospital admission or life-threatening attack; lung function: FEV1 > 80% of predicted value, morning PFR > 90% of recent best.
Moderate: symptoms most days, but nocturnal and early morning symptoms less than once weekly; usually no previous hospital admission or life-threatening attack; lung function: FEV1 60%–80% of predicted value, morning PFR 80%–90% of recent best.
Severe: daily symptoms, with nocturnal and early morning symptoms more than once weekly; usually previous hospital admissions and/or previous life-threatening attack; lung function: FEV1 < 60% of predicted value, morning PFR < 80% of recent best.
FEV1 = forced expiratory volume in 1 second. PFR = peak flow rate.
3 Proposed pathophysiological links between the upper and lower airways
The mechanism by which communication occurs between the upper and lower airways is suggested to be via a bone marrow-derived systemic inflammatory response (Box 4).
Allergic rhinitis has adverse effects on the lower airway by the promotion of breathing through the mouth.
A neurogenic (nasobronchial) reflex has also been suggested (eg, transient bronchoconstriction resulting from irritant stimulation of nasal mucosa).
Secretions (liquid or gaseous) may drip or diffuse from the upper airway to the lower airway.
High nasal nitric oxide concentrations (up to 100 times greater than in orally exhaled air) are thought to have antiviral, bacteriostatic and bronchodilator effects on the lower airway.
5 Case scenario*
Samter’s triad (aspirin sensitivity with nasal polyposis and asthma) affects 4%–11% of patients with asthma and is considered to be mediated by overproduction of leukotrienes via the cyclooxygenase and lipoxygenase pathways. While the mechanisms by which aspirin desensitisation benefits patients are incompletely understood, aspirin therapy results in reduced leukotriene levels.12 A combination of aspirin desensitisation and anti-leukotriene therapy could be expected to provide added benefit, but there is currently only Level III evidence† for this approach.13
* This is a fictional case scenario based on similar real-life cases. † National Health and Medical Research Council levels of evidence.14
6 Efficacy of therapies for rhinitis and asthma
* – = no effect; +++ = the most effective. † Based on National Health and Medical Research Council levels of evidence.14 |
|||||||||||||||
7 Evidence-based practice tips*
Coexistence of rhinitis and asthma is common and should be looked for in all patients (Level IV).
Allergen immunotherapy is effective for treating both rhinitis and asthma (Level I).
Aspirin desensitisation can be an effective therapy in selected patients with Samter’s triad (aspirin sensitivity, nasal polyposis and asthma). Because of the risks involved, any decisions about aspirin desensitisation should only be made after specialist review (Level II).
Avoidance of house dust mite allergen can not currently be recommended to improve asthma or rhinitis (Level I).
* Based on National Health and Medical Research Council levels of evidence.14
- Janet Rimmer1,2
- John W Ruhno2,3
- 1 Department of Thoracic Medicine, St Vincent’s Clinic, Sydney, NSW.
- 2 Department of Allergy, Royal North Shore Hospital, Sydney, NSW.
- 3 Department of Allergy, Immunology and Infectious Diseases, The Children’s Hospital at Westmead, Sydney, NSW.
Janet Rimmer has received honoraria from GlaxoSmithKline for conducting clinical trials at the Woolcock Institute of Medical Research.
- 1. Corren J, Togias A, Bousquet J, editors. Lung biology in health and disease. Volume 181: upper and lower respiratory disease. New York: Marcel Dekker, 2003.
- 2. Bousquet J, Jacot W, Vignola AM, et al. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol 2004; 113: 43-49.
- 3. Kay AB. Concepts of allergy and hypersensitivity. In: Kay AB, editor. Allergy and allergic diseases. Oxford: Blackwell Science, 1997.
- 4. Management of allergic rhinitis and its impact on asthma: pocket guide. Asthma workshop report in collaboration with the World Health Organization. Geneva: WHO, 2001.
- 5. National Asthma Council Australia. Asthma management handbook 2002. Melbourne: National Asthma Council Australia Ltd, 2002.
- 6. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol 2003; 111: 1171-1183.
- 7. McCusker CT. Use of mouse models of allergic rhinitis to study the upper and lower airway link. Curr Opin Allergy Clin Immunol 2004; 4: 11-16.
- 8. Gaga M, Lambrou P, Papageorgiou N, et al. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy 2000; 30: 663-669.
- 9. Braunstahl GJ, Kleinjan A, Overbeek SE, et al. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med 2000; 161: 2051-2057.
- 10. Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med 2001; 164: 858-865.
- 11. Denburg JA, Sehmi R, Saito H, et al. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol 2000; 106 (5 Suppl): S242-S246.
- 12. Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol 2006; 4 Sep [online].
- 13. Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2003; 111: 180-186.
- 14. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Canberra: NHMRC, 2000. http://www.nhmrc.gov.au/publications/_files/cp69.pdf (accessed Jul 2006).
- 15. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy 2005; 35: 598-601.
- 16. Hurst JR, Wilkinson TM, Perera WR, et al. Relationships among bacteria, upper airway, lower airway, and systemic inflammation in COPD. Chest 2005; 127: 1219-1226.
- 17. Hurst JR, Perera WR, Wilkinson TM, et al. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173: 71-78.
- 18. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998; 351: 1225-1232.
- 19. Toelle BG, Ng K, Belousova E, et al. Prevalence of asthma and allergy in schoolchildren in Belmont, Australia: three cross sectional surveys over 20 years. BMJ 2004; 328: 386-387.
- 20. Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002; 109: 419-425.
- 21. Toelle BG, Xuan W, Peat JK, Marks GB. Childhood factors that predict asthma in young adulthood. Eur Respir J 2004; 23: 66-70.
- 22. Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol 2001; 107: 73-80.
- 23. Tosca MA, Cosentino C, Pallestrini E, et al. Improvement of clinical and immunopathologic parameters in asthmatic children treated for concomitant chronic rhinosinusitis. Ann Allergy Asthma Immunol 2003; 91: 71-78.
- 24. Taramarcaz P, Gibson PG. Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis. Cochrane Database Syst Rev 2003; (3): CD003570.
- 25. Grieff L, Andersson M, Svensson C, et al. Effects of orally inhaled budesonide in seasonal allergic rhinitis. Eur Respir J 1998; 11: 1268-1273.
- 26. Stelmach R, do Patrocinio T Nunes M, Ribeiro M, Cukier A. Effect of treating allergic rhinitis with corticosteroids in patients with mild-to-moderate persistent asthma. Chest 2005; 128: 3140-3147.
- 27. Adams RJ, Fuhlbrigge AL, Finkelstein JA, Weiss ST. Intranasal steroids and the risk of emergency department visits for asthma. J Allergy Clin Immunol 2002; 109: 636-642.
- 28. Grant JA, Nicodemus CF, Findlay SR, et al. Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol 1995; 95: 923-932.
- 29. Warner JO; ETAC Study Group. Early Treatment of the Atopic Child. A double-blinded, randomized, placebo-controlled trial of cetirizine in preventing the onset of asthma in children with atopic dermatitis: 18 months’ treatment and 18 months’ posttreatment follow-up. J Allergy Clin Immunol 2001; 108: 929-937.
- 30. Greenfeder S, Umland SP, Cuss FM, et al. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res 2001; 2: 71-79.
- 31. Donnelly AL, Glass M, Minkwitz MC, Casale TB. The leukotriene D4-receptor antagonist, ICI 204,219, relieves symptoms of acute seasonal allergic rhinitis. Am J Respir Crit Care Med 1995; 151: 1734-1739.
- 32. Thien FC. Leukotriene receptor antagonist drugs for asthma. Med J Aust 1999; 171: 378-381.
- 33. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy 2005; 35: 408-416.
- 34. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med 2006; 354: 2689-2695.
- 35. Alves B, Sheikh A, Hurwitz B, Durham SR. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2000; (1): CD001936.
- 36. Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev 2003; (4): CD001186.
- 37. Pajno GB, Barberio G, De Luca F, et al. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy 2001; 31: 1392-1397.
- 38. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol 2002; 109: 251-256.
- 39. Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol 2004; 114: 851-857.
- 40. Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma. Cochrane Database Syst Rev 2004; (4): CD001187.
- 41. O’Connor GT. Allergen avoidance in asthma: what do we do now? J Allergy Clin Immunol 2005; 116: 26-30.
- 42. Peat JK, Mihrshahi S, Kemp AS, et al. Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the Childhood Asthma Prevention Study. J Allergy Clin Immunol 2004; 114: 807-813.





Abstract
United airway disease is characterised by inflammation of the respiratory tract, in which asthma and rhinitis are the upper and lower respiratory tract manifestations, respectively, of the same disease process.
Irrespective of cause, the upper and lower respiratory tract manifestations are characterised by a systemic inflammatory response.
Patients with rhinitis or asthma should always be assessed for coexistent disease in the reciprocal area.
Treatment of upper airway disease can modify the severity of lower airway disease and vice versa.
The potential for early treatment of allergic rhinitis to prevent progression to asthma merits further study.