Vaccine manufacturers have been developing prototype vaccines against influenza A/H5N1 in anticipation of an emergent pandemic, but initial control measures such as social distancing and antiviral prophylaxis will be important because of the anticipated delays in vaccine production. A prototype vaccine will not be a perfect match for an emergent virus, as we will not know the exact antigenic constitution of the pandemic strain until the pandemic actually occurs. However, a prototype vaccine may provide a degree of protection and be useful as a stop-gap measure until a matched vaccine is produced, 3–6 months into the pandemic.1
Influenza vaccines are currently grown in fertile hens eggs, making it a slow and labour-intensive production process. The highly pathogenic avian H5N1 virus may be lethal to or grow poorly in eggs, thus compromising production capacity. Furthermore, it is likely that two doses of pandemic vaccine at a higher dose than seasonal vaccine will be required to optimise protection in humans.2-4 Minimising the amount of viral antigen needed per dose of vaccine to compensate for these factors will be essential to provide sufficient yields of life-saving vaccines. Early in 2006, the United States government committed more than US$1 billion to support research by five different pharmaceutical companies into improving cell culture as an alternative to cultivation in fertile eggs.
Late in 2005, in the US, Sanofi Pasteur conducted an early trial of an unadjuvanted vaccine for H5N1, derived from a genetically modified strain. In this trial, 90 μg (six times the standard influenza vaccine dose) of H5 haemagglutinin was required to induce accept-able immunogenicity2 — a worrying finding if there is to be any hope of producing enough vaccine to protect the wider population. Reassuringly, vaccination was well tolerated.
By contrast, in July 2006, the Chief Executive Officer of GlaxoSmithKline claimed that their new, adjuvanted preparation was immunogenic in humans given only 3.8 μg (a quarter of the standard dose). This is a greater than 20-fold turnaround in potency if the results are reproducible.5 Was it just an effect of the adjuvant? A surprising number of variables may differ between vaccine studies, in addition to adjuvant use and type. These factors include:
Antigen content;
Use of whole inactivated virion or detergent-split virus preparation;
Growth in eggs or cell culture;
Sex and age of subjects (the GlaxoSmithKline trial had a ceiling age of 40 years, whereas the Sanofi Pasteur trial included a large proportion of subjects aged 40–65 years, in whom immunogenicity will be less);
Prior exposure to seasonal human influenza or vaccination; and
More subtle differences, like whether methods include recruiting subjects with prior positive involvement in vaccination trials (a variant of the “healthy volunteer effect”) and the type of assay used to measure protection.
A recent European trial compared doses of 7.5 μg, 15 μg and 30 μg of haemagglutinin with or without aluminium hydroxide adjuvant in 300 healthy volunteers aged 18–40 years. This study found that a two-dose regimen of 30 μg induced the highest response, with adjuvanted vaccine being more immunogenic than the non-adjuvanted vaccine, but only at the highest dose.3 Australian manufacturer CSL Ltd has also completed trials of an H5N1 vaccine candidate, but the results are not available. Other vaccine research in ferrets, the closest animal model of relevance to humans, shows that a two-dose regimen of vaccine in an immunologically naïve population is not only immunogenic against the target virus, but also provides cross-protection against antigenically distinct H5N1 strains.4 Cross-protection mediated by cellular immune responses to internal conserved antigens (called heterosubtypic protection) may also play a role, but more research in this area is needed.
The Australian Government decided in 2005 to stockpile up to five million doses of prototype H5N1 vaccine, provided evidence is produced of safety and efficacy (ie, likely protection based on antibody responses). A prototype vaccine will not be a perfect match for an emergent virus (remembering that the pandemic virus may not even be H5N1). However, it may be of some benefit as a stop-gap measure until a matched vaccine is produced.4 The Australian Government has also announced it would acquire up to 50 million doses of “pandemic strain” vaccine from two suppliers (CSL; and Sanofi Pasteur, Paris), if a pandemic occurs.6
The WHO advised in August 2006 that the choice of H5N1 strains for development of candidate vaccines should be representative of the distinct groups (clades) of viruses that have been afflicting humans recently; this equates to recommending that in addition to clade 1 H5N1 virus (eg, the 2004 Vietnam strain used in trials reported above), examples of clade 2 (a variant of H5N1 now circulating in Indonesia) should also be included. The recent 2005–2006 outbreaks in Indonesia due to clade 2 H5N1 viruses have already resulted in more than 50 human deaths and afflicted poultry in 28 of Indonesia’s 33 provinces.7 This raises the need for a whole new swathe of studies to assess safety, immunogenicity, priming, cross-reactivity and cross-protection of vaccines against a clade 2 H5N1 strain.
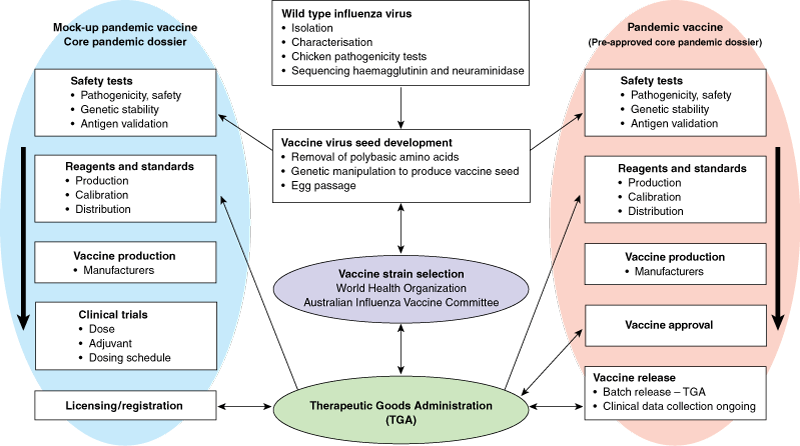
Ideally, the mock-up vaccine would be produced in the same way as intended for the pandemic vaccine, whether from cell culture or egg; comparisons would be made between whole virion and split or subunit vaccines; it would have similar antigen content as any future pandemic vaccine; and it would have the same adjuvant system (if used) as the future pandemic vaccine. The dossier containing pre-clinical (quality, safety and immunogenicity) and clinical data would be submitted to the TGA for evaluation. In the event of a pandemic, an application for a product variation would be submitted, containing the manufacturing and quality control data relating to the pandemic influenza strain and a commitment from the sponsor to gather clinical information during the pandemic (Box). This would permit a faster approval process.
Whole formalin-inactivated virions;
Highly attenuated or replication incompetent influenza viruses, including those lacking or defective in critical components necessary for efficient in-vivo growth, such as the nuclear export protein,9 the interferon antagonist protein NS110 or the M2 ion channel;11 and
Influenza virosomes, which are reconstituted viral envelopes devoid of the viral genome.12
Strategies that do not rely on egg-grown virus and could potentially supplement these include:
The merit in vaccinating now with a current H5N1 virus isolate, despite the likelihood of its significant variation from a future pandemic strain, is under debate. The current vaccine strain may induce sufficient cross-reactive antibody to curtail, although not effectively prevent, infection by the pandemic strain.4
Future vaccines may depend on strategies that also target the internal conserved proteins of the virus and elicit heterosubtypic immunity (ie, common to all influenza A viruses). Such broadly cross-reactive responses, not induced by current inactivated virus preparations, can be mediated by CD8+ cytotoxic T cells that kill virus-infected cells and secrete antiviral cytokines. These vaccines cannot prevent infection, but lessen the severity and duration of disease and reduce viral shedding. DNA vaccines encoding genes for internal proteins no doubt work principally by this method. Other strategies involving delivery of conserved CD8+ T cell epitopes16 and the use of adjuvants that may boost cross-reactive T cell responses are the subject of NHMRC-funded studies. The advantage of boosting heterosubtypic immunity is that vaccines can be delivered without prior knowledge of the emerging strain.
- Robert Booy1
- Lorena E Brown2
- Gary S Grohmann4
- C Raina MacIntyre1
- 1 National Centre for Immunisation Research and Surveillance, The Children's Hospital at Westmead, Sydney, NSW.
- 2 Department of Microbiology and Immunology, University of Melbourne, Melbourne, VIC.
- 3 Therapeutic Goods Administration Laboratories, Canberra, ACT.
- 4 Discipline of Infectious Diseases and Immunology, Central Clinical School, Department of Medicine, University of Sydney, Sydney, NSW.
Robert Booy has received support to attend scientific meetings from CSL, Sanofi, Roche and Wyeth. Raina MacIntyre is an investigator on an ARC Linkage grant in which Roche is a partner, and has had a conference registration fee paid by CSL.
- 1. Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines. Wkly Epidemiol Rec 2006; 81: 328-330.
- 2. Treanor JJ, Campbell JD, Zangwill KM, et al. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354: 1343-1351.
- 3. Bresson J-L, Perronne C, Launay O, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006; 367: 1657-1664.
- 4. Govorkova EA, Webby RJ, Humberd J, et al. Immunization with reverse-genetics–produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis 2006; 194: 159-167.
- 5. GSK’s H5N1 flu vaccine achieves high response at low dose. PharmaWeek 2006; 26 Jul. http://www.pharmaweek.com/TopNews/GSK's%20H5N1.asp (accessed Oct 2006).
- 6. Abbott T. Infectious diseases conference, pandemic preparedness [speech notes]. 2 May 2005. http://www.health.gov.au/internet/ministers/publishing.nsf/Content/health-mediarel-yr2005-ta-abbsp020505.htm (accessed Aug 2006).
- 7. World Health Organization. Avian influenza — situation in Indonesia — update 35. 3 October 2006. http://www.who.int/csr/don/2006_10_03/en/index.html (accessed Oct 2006).
- 8. Global Advisory Committee on Vaccine Safety, 6–7 June, 2006. Wkly Epidemiol Rec 2006; 81: 273-276.
- 9. Watanabe T, Watanabe S, Neumann G, et al. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J Virol 2002; 76: 767-773.
- 10. Talon J, Salvatore M, O’Neill RE, et al. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc Natl Acad Sci U S A 2000; 97: 4309-4314.
- 11. Watanabe T, Watanabe S, Kida H, Kawaoka Y. Influenza A virus with defective M2 ion channel activity as a live vaccine. Virology 2002; 299: 266-270.
- 12. Huckriede A, Bungener L, Stegmann T, et al. The virosome concept for influenza vaccines. Vaccine 2005; 23 Suppl 1: S26-S38.
- 13. Brands R, Visser J, Medema J, et al. Influvac: a safe Madin Darby Canine Kidney (MDCK) cell culture-based influenza vaccine. Dev Biol Stand 1999; 98: 93-100.
- 14. Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol 2005; 18: 244-251.
- 15. Kodihalli S, Goto H, Kobasa DL, et al. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol 1999; 73: 2094-2098.
- 16. Jackson DC, Lau YF, Le T, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A 2004; 101: 15440-15445.





Abstract
Prototype vaccines against influenza A/H5N1 may be poorly immunogenic, and two or more doses may be required to induce levels of neutralising antibody that are deemed to be protective. The actual levels of antibody required to protect against a highly pathogenic virus that potentially can spread beyond the large airways is unknown.
The global capacity for vaccine manufacture in eggs or tissue culture is considerable, but the number of doses that can theoretically be produced in a pandemic context will only be sufficient for a small fraction of the world’s population, even less if a high antigen content is required.
The safety of new pandemic vaccines should be addressed in an internationally coordinated way.
Steps are underway through the Therapeutic Goods Administration to evaluate mock-up vaccines now, so that the time to registration of a new product can be minimised.
It will be 3–6 months into the pandemic before an effective vaccine becomes available, so other control measures will be important in the early stages of a pandemic.
The primary goal of a pandemic influenza vaccine must be to prevent death, and not necessarily to prevent infection.