Non-selective non-steroidal anti-inflammatory drugs (NSAIDs) provide effective management of pain and inflammation, but are associated with the formation of peptic ulcers and an increased risk of peptic ulcer haemorrhage and perforations (“serious gastrointestinal events”) in the range of 0.3% to 2.5% per year.1,2 The background rate of serious gastrointestinal (GI) events has been estimated as 0.1%–0.2% per year in non-exposed people.3,4 This may be higher depending on co-existing factors such as smoking, use of anticoagulants5 and older age. However, NSAID-related and background serious GI event rates from different studies are not informative for comparison. Studies comparing NSAID users against well-matched controls are more informative, and show NSAIDs to be associated with a 1.5–7.2-fold increase in serious GI events, compared with controls, across various agents.6-8 Thus, for patients who require long-term therapy, clinicians must weigh the risk of serious GI events relative to other risks.9-12
The hypothesis that animals develop mucosal tolerance to the GI-toxic effects of NSAIDs has been proposed repeatedly.8,13,14 However, the relevance of this to humans and serious GI events is unclear.15-18 Therefore, we performed a quantitative systematic review to determine whether the occurrence of serious GI events reduces over time in clinical studies of long-term NSAID use. Serious GI events were defined as upper GI ulcers that were complicated by haemorrhage, perforations, obstructions or hospitalisation.
study sample included patients exposed to prescription non-selective NSAIDs for a duration greater than 6 months;
“serious GI event” incidence or risk was reported, by duration of NSAID exposure;
events occurred during current use of medication; and
the study was published in full in English, French or German.
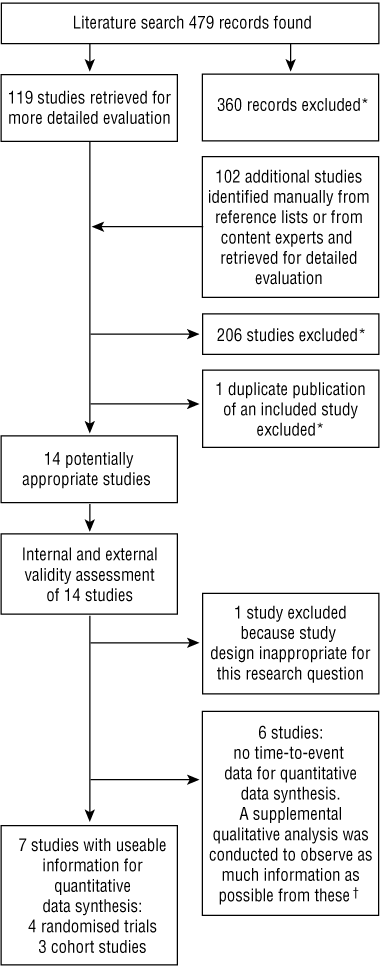
Using the same search criteria, a high-specificity search of Current Contents, EMBASE, MEDLINE and Derwent Drug File databases was conducted in July 2002 (limitations: humans, 1999–2002). In this manner, relevant observational studies and further randomised trials were identified either directly (back to 1999) or indirectly (by manual search of systematic reviews from 1999–2002, representing the NSAID literature back to the 1960s). Abstracts of 479 search results were reviewed; 360 records were excluded for not fulfilling the basic criteria, and 119 records plus an additional 102 records identified from manual searches were retrieved for more detailed evaluation (total, 221) (Box 1).
When retrospective cohort studies using the same health care databases (eg, Tennessee Medicaid, UK General Practice Research Database, Tayside, or ARAMIS) were identified, only one study per database was selected; the rest5,6,19-30 were excluded to avoid potentially redundant patient populations. Meta-analyses were excluded; however, any otherwise unidentified original published studies cited in meta-analyses were retrieved for evaluation against our search criteria. Exclusions were logged with a reason (log available from authors as Appendix 1).
Fourteen studies (represented by 15 publications) were identified as potentially relevant.
Individual studies were reviewed to evaluate the presence of a valid statistical association. Key parameters affecting internal and external validity were assessed according to standard epidemiological principles and Australian government guidelines.31-34 Details are available from the authors as Appendix 2. Based on the assessments, seven studies were included in the quantitative data synthesis. These were stratified into two groups according to study design:
Group 1. Four randomised trials presenting time-to-event data.35-38 These investigated patients who were using NSAIDs on a regular daily basis and who were subject to protocol-driven constraints (ie, patients were not allowed to switch NSAIDs or to take concomitant anti-ulcer medication; patients were withdrawn after a serious GI event or other monitored adverse events).
Group 2. Three cohort studies presenting time-to-event data: one prospective study2 and two registry studies.4,39 It is likely that these studies included some patients who used NSAIDs on an “as needed” (prn) basis — detailed compliance information was unavailable.
Box 2 and Box 3 summarise the validity weaknesses identified for the two groups.
Six observational studies,40-45 classified as Group 3, were excluded for lack of time-to-event data for data synthesis. These reported relative risk or excess risk for NSAID exposure versus non-exposure by duration-of-exposure categories, and were reviewed in a supplemental analysis. Details are available from the authors as Appendix 3.
An additional case–control study15 nested within a cohort of patients exposed to NSAIDs for up to 1 year was excluded. Odds ratios were not reported, but serious GI bleed rates over time were, so it potentially qualified for quantitative data synthesis inclusion. However, unlike the included studies, that study based rates on estimated exposure duration (derived from a selected subgroup of patients who did not have a serious GI event), rather than actual exposure data from the entire cohort of exposed patients. This led to concerns about bias, as selected patients without bleeds were unlikely to be representative of patients with bleeds. Furthermore, information on numbers at risk over time was not available for that study.
Data for validity assessment criteria and patient characteristics were extracted from the articles (variables available on request). Outcomes data for serious GI event incidence at specific times were extracted where available, or otherwise were taken from an electronic citation,46 email correspondence (Dr M Mamdani, Scientist, Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada) and unpublished reports (G Singh, Analysis of a cohort of long-term diclofenac users, 2003; S Morant, Statistician, Cygnus Biostatistics Ltd, Analysis of NSAID exposure duration and GI haemorrhage rates using the UK GP Database, 2002; GD Searle & Co, Final Report for the CLASS trial, 2000). If multiple serious GI event outcome definitions were reported, data were extracted for the strictest definition. Data were only extracted for patients taking non-selective NSAIDs (ie, data from placebo, COX-2 specific inhibitor, or diclofenac/misoprostol treatment arms found in some studies were outside the scope of our review).
For Group 1 and 2 studies separately, a combined analysis across studies and time points using a generalised estimating equations linear regression model47 (a form of meta-regression) was conducted using SAS software version 8 (SAS Institute Inc, Cary, NC, USA). The outcome variable was the estimated annualised probability of having an event, for each time point in each study. This was estimated from the conditional probability of having a serious ulcer complication in a specified time interval, given that no event was experienced before the start of that interval, and was derived from the published cumulative probability estimates for each study. The model included time (in months), study and treatment group as explanatory variables. Change in risk was evaluated by estimating and testing the significance of the regression line slope. Weighted analyses were conducted using the inverse variance method, with variance estimated from Greenwood’s formula.48 A sensitivity analysis using inverse standard error weighting was conducted to verify robustness of the estimates. As data were too limited to perform valid heterogeneity testing within the regression model, pre-assessment of heterogeneity was done (details available from the authors as Appendix 4). Lastly, adequacy of the linear model was assessed by including higher-order terms in the model.
The studies investigated a wide selection of NSAIDs taken in usual clinical doses. One study included aspirin at 1000 mg/day,37 a dose that is standard for pain management but higher than commonly used in Australia for antithrombotic treatment in patients with myocardial infarction. The studies measured the occurrence of upper GI ulcer bleeds and perforations. In addition to ulcer bleeds, two trials37,38 and one observational study43 included hospitalised symptomatic ulcers in the outcome definition.
All studies sampled adult community populations of NSAID users. They included patients with osteoarthritis36 and rheumatoid arthritis,2,35,36,38 unspecified NSAID users,41,42,44,45 or subgroups (> 65 years;4 > 40 years;39,40 and low-income elderly43). NSAID use was for pain treatment, or, if unspecified, was assumed most likely for pain. One study enrolled only patients with myocardial infarction.37 Most patients had taken NSAIDs before study commencement, but one cohort study specifically included NSAID-naïve patients.4
Of the randomised trials, the AMIS trial37 included 100% long-term users (> 6 months) and the VIGOR,35 Neustadt38 and CLASS36 trials included more than 50% long-term users. The prospective cohort study by Singh and Triadafilopoulos2 included more than 50% long-term users. Although the registry cohort and case–control studies were larger, they included a lower proportion of long-term users (< 12% in Mamdani et al,4 12% in UKGPRD,39 and < 18% in Mellemkjaer et al42). Information about the proportion of long-term users was not available for the other three studies.
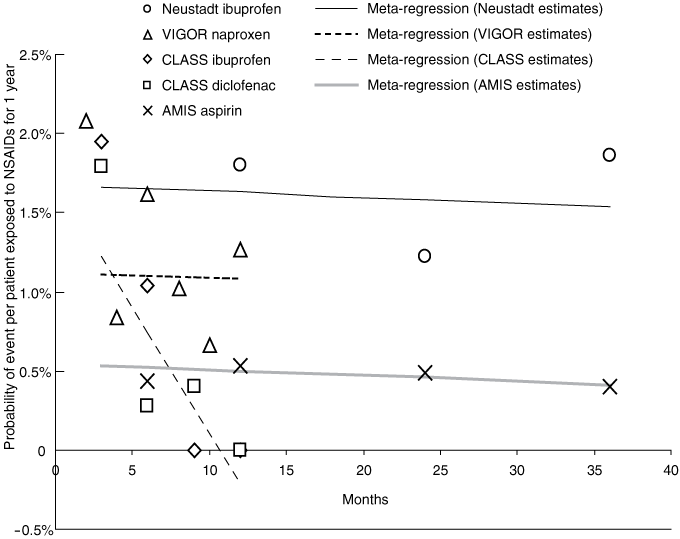
The results of the individual studies are provided in Box 2 and in the Kaplan–Meier curves in the original articles. Four randomised trials provided Kaplan–Meier time-to-event data from seven non-selective NSAID groups (aspirin 1000 mg/day, na-proxen 1000 mg/day, diclofenac 150 mg/day, two arms with ibuprofen 2400 mg/day, and two arms with etodolac 300 mg/day and 1000 mg/day). Five groups were included in the pooled regression model; the etodolac arms38 were excluded as time-dependent analysis was not considered valid with only two serious GI events per arm.
When randomised trial data were pooled, a small significant reduction in risk of − 0.005% (95% CI, − 0.008% to − 0.001%) per month was seen (Box 4). Three sensitivity analyses were performed. The first addressed the finding that the slope of the CLASS trial data was significantly different to that of the other studies, mainly driven by the ibuprofen group. The analysis was re-run allowing different slopes for the CLASS study versus the other studies. The latter studies showed a small decline in annualised risk of serious GI events of − 0.004% (95% CI, − 0.005% to − 0.003%) per month. The disparate CLASS study showed a significant decline in annualised risk of serious GI events of − 0.16% (95% CI, − 0.223% to − 0.096%) per month of exposure. The second sensitivity analysis again allowed different slopes for the CLASS study versus the others, and excluded the AMIS study (because the patient population was very different to those in the other trials). This showed a non-significant decline in annualised risk of − 0.018% (95% CI, − 0.04% to 0.001%) per month for the VIGOR and Neustadt studies combined. Results were similar when sensitivity analysis was conducted using the alternate weighting method.
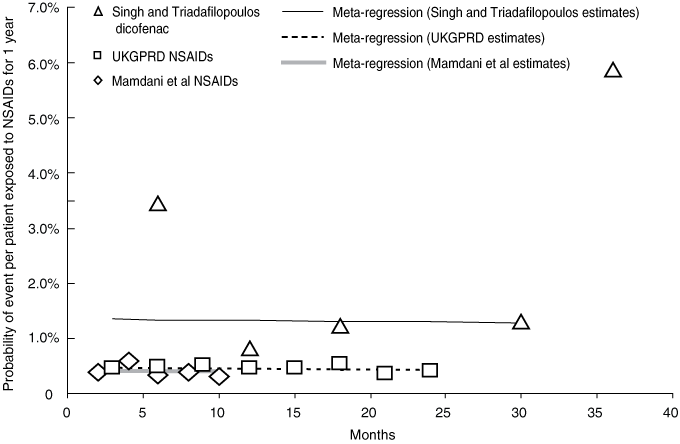
The results of the individual studies are summarised in Box 3. For the pooled analysis, the NSAID cohort from the study by Singh and Triadafilopoulos2 was excluded because information on patient numbers over time was not retrievable. Time-to-event data from the diclofenac cohort of the study by Singh and Triadafilopoulos and data from the other two cohort studies were pooled. The slope of the combined regression line shows no significant decline in the annualised probability of serious GI events over time (Box 5). Results were similar when sensitivity analysis was conducted using the alternate weighting method.
Our findings are difficult to compare with previously published risk-duration meta-analyses,7,49-51 primarily because previous meta-analyses combined serious and non-serious GI events, and were heavily weighted for short-term studies (7–90 days). Where long-term (> 6 month) studies were cited,51 the meta-analyses did not meet our criteria for time-to-event data, instead comparing short-term and long-term NSAID users from different studies. These analyses showed no trend for decreasing risk over study durations of 60 days and longer.
This is the first time, to our knowledge, that all available time-to-event data from long-term NSAID studies have been reviewed. Our findings are consistent with three published time-to-event meta-analyses of nabumetone data and non-selective NSAID data (from comparative studies against rofecoxib).52-54
The primary limitation of our study was the small number of long-term studies. Our analysis is a form of meta-regression, which should generally not be considered when there are fewer than 10 trials.55 However, because our analysis had multiple time points per study, this guidance is not as relevant. Detailed discussion of other limitations (eg, the studies, except two, were not designed a priori to evaluate this question; use of high-specificity literature search methods) is available on request. The small number of studies and other limitations mainly affected the precision of the results, but overall, loss of precision would not change the conclusions regarding the nature, direction (slight downward slope) and marginal clinical significance of the risk-over-time relationship.
Our review provides an overview of the evidence to 2002. Although the trends observed in this analysis may suffice for the purposes of clinical practice, further large trials are needed to address this question with optimal statistical rigour. We are aware of one randomised trial that was completed after the 2002 data cut-off for our project. The trial included very large samples of naproxen and ibuprofen users, and reported the cumulative occurrence of serious GI events over 1 year.17 Our analysis could not be updated to include the trial because necessary patient numbers at various time points are not publicly available. However, the published Kaplan–Meier results support our findings of consistent risk over time, leading to an accumulation of serious risk over the long term.17
If future studies are conducted, these should, if feasible, minimise the effect of rare-event bias56,57 (which can be amplified over time for pain agents with tolerability issues) and depletion of susceptibles, maximise the numbers of patients remaining in the study long term, and capture potentially important confounding factors such as anticoagulant use, intermittent versus regular therapy, and whether patients continued NSAID treatment after a serious GI event. These were sources of uncertainty in this review and raised new research questions.
2 Time-to-event results from randomised trials
3 Time-to-event results from cohort studies
Received 14 September 2005, accepted 22 August 2006
- Delia Schaffer1
- Timothy Florin2,3
- Craig Eagle1
- Ian Marschner1
- Gurkirpal Singh4
- Mendel Grobler1
- Chris Fenn1
- Manjula Schou1
- Kathleen M Curnow1
- 1 Pfizer Australia, Sydney, NSW.
- 2 Department of Medicine, University of Queensland, Brisbane, QLD.
- 3 Gastroenterology Unit, Mater Health Services Adult Hospital, Brisbane, QLD.
- 4 School of Medicine, Division of Gastroenterology and Hepatology, Stanford University, Stanford, Calif, USA.
Gurkirpal Singh has received research and educational grants, consultancy fees and speaker fees from Pfizer, and has collaborated with Pfizer in gastroenterology research on several occasions. Delia Schaffer, Mendel Grobler, Manjula Schou and Ian Marschner are full-time employees of Pfizer Australia. Craig Eagle was employed by Pfizer Australia when this review was conducted, and is now employed by Pfizer Inc (New York, USA). Kathleen Curnow was a full-time employee of Pharmacia Australia and then Pfizer Australia when this review was conducted. Timothy Florin has been a correspondent to Digestive Disease Week (USA) for gastroenterology updates, partly supported by Pfizer. Chris Fenn is a part-time consultant to Pfizer Australia. Manjula Schou, Ian Marschner and Chris Fenn hold stock and/or stock options in Pfizer. This review was conducted as routine employment duty to support the Pharmaceutical Benefits Advisory Committee submission for celecoxib. There was no specific monetary funding for this project. Authors not employed by Pfizer participated on a volunteer basis. No person at Pfizer other than the authors had any role in the study design, data collection, analysis, interpretation, writing or publication of this article.
- 1. Goldstein JL, Silverstein FE, Agrawal NM, et al. Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. Am J Gastroenterol 2000; 95: 1681-1690.
- 2. Singh G, Triadafilopoulos G. Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl 1999; 26: 18-24.
- 3. Hernandez-Diaz S, Garcia Rodriguez LA. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol 2002; 55: 157-163.
- 4. Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 2002; 325: 624-629.
- 5. Shorr RI, Ray WA, Daugherty JR, et al. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorraghic peptic ulcer disease. Arch Intern Med 1993; 153: 1665-1670.
- 6. Garcia Rodriguez LA, Hernandez-Diaz S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology 2001; 12: 570-576.
- 7. Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996; 312: 1563-1566.
- 8. Langman MJ, Brooks P, Hawkey CJ, et al. Non-steroidal anti-inflammatory drug associated ulcer: epidemiology, causation and treatment. J Gastroenterol Hepatol 1991; 6: 442-449.
- 9. Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case–control study. Lancet 2005; 365: 475-481.
- 10. Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005; 330: 1366-1369.
- 11. Hudson M, Hugues R, Pilote L. Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory drugs: population based study. BMJ 2005; 330: 1370-1373.
- 12. Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med 2005; 165: 978-984.
- 13. Shorrock CJ, Prescott RJ, Rees WD. The effects of indomethacin on gastroduodenal morphology and mucosal pH gradient in the healthy human stomach. Gastroenterology 1990; 99: 334-339.
- 14. Skeljo MV, Giraud AS, Yeomans ND. Adaptation of rat gastric mucosa to repeated doses of non-salicylate non-steroidal anti-inflammatory drugs. J Gastroenterol Hepatol 1992; 7: 586-590.
- 15. Carson JL, Strom BL, Soper KA, et al. The association of nonsteroidal anti-inflammatory drugs with upper gastrointestinal tract bleeding. Arch Intern Med 1987; 147: 85-88.
- 16. Elliott SL, Yeomans ND, Buchanan RR, Smallwood RA. Efficacy of 12 months’ misoprostol as prophylaxis against NSAID-induced gastric ulcers. A placebo-controlled trial. Scand J Rheumatol 1994; 23: 171-176.
- 17. Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004; 364: 665-674.
- 18. Paulus HE. Government Affairs. FDA Arthritis Advisory Committee meeting: risks of agranulocytosis/aplastic anemia, flank pain, and adverse gastrointestinal effects with the use of nonsteroidal antiinflammatory drugs. Arthritis Rheum 1987; 30: 593-595.
- 19. Fries JF, Williams CA, Bloch DA, Michel BA. Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models. Am J Med 1991; 91: 213-222.
- 20. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994; 343: 769-772.
- 21. Garcia Rodriguez LA, Walker AM, Perez Gutthann S. Nonsteroidal anti-inflammatory drugs and gastrointestinal hospitalizations in Saskatchewan: a cohort study. Epidemiology 1992; 3: 337-342.
- 22. Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med 1988; 109: 359-363.
- 23. Griffin MR, Piper JM, Daugherty JR, et al. Non-steroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114: 257-263.
- 24. McMahon AD, Evans JM, White G, et al. A cohort study (with resampled comparator groups) to measure the association between new NSAID use prescribing and upper gastrointestinal hemorrhage and perforation. J Clin Epidemiol 1997; 50: 351-356.
- 25. Perez Gutthann S, Rodriguez LA, Raiford DS. Individual non-steroidal anti-inflammatory drugs and the risk of hospitalisation for upper gastrointestinal bleeding and perforation in Saskatchewan: a nested case–control study. Pharmacoepidemiol Drug Saf 1994; 3: S63.
- 26. Singh G, Fries JF, Spitz PW, et al. Toxic effects of azathioprine in rheumatoid arthritis: a national post-marketing perspective. Arthritis Rheum 1989; 32: 837-841.
- 27. Singh G, Fries JF, Spitz PW, et al. Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol 1991; 18: 188-194.
- 28. Singh G, Ramey DR, Morfeld D, et al. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis: a prospective observational cohort study. Arch Intern Med 1996; 156: 1530-1536.
- 29. Singh G, Terry R, Ramey DR, et al. Epidemiology of serious NSAID-related complications: a prospective multivariate lifetable analysis. Arthritis Rheum 1997; 40 (9 Suppl): S213.
- 30. Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med 1998; 105: 31S-38S.
- 31. Christie D, Gordon I, Heller H. Randomised controlled trials and longitudinal studies. In: Madjar N, editor. Epidemiology: an introductory text for medical and other health science students. 2nd ed. Sydney: UNSW Press, 1997: 93, 94, 64-78.
- 32. Australian Government Department of Health and Ageing. Appendices Q and R. In: Guidelines for the pharmaceutical industry on preparation of submissions to the Pharmaceutical Benefits Advisory Committee including major submissions involving economic analyses. Canberra: Department of Health and Ageing, 2002. http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pbs-general-pubs-guidelines-content.htm (accessed Sep 2002).
- 33. Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12.
- 34. Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999; 354: 1896-1900.
- 35. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000; 343: 1520-1528.
- 36. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib versus nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the Celecoxib Long-Term Arthritis Safety Study: a randomised controlled trial. JAMA 2000; 284: 1247-1255.
- 37. Kurata JJ, Abbey DE. The effect of chronic aspirin use on duodenal and gastric ulcer hospitalizations. J Clin Gastroenterol 1990; 12: 260-266.
- 38. Neustadt DH. Double blind evaluation of the longterm effects of etodolac versus ibuprofen in patients with rheumatoid arthritis. J Rheumatol Suppl 1997; 47: 17-22.
- 39. MacDonald TM, Morant SV, Goldstein JL, et al. Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxib, and older, non-specific non-steroidal anti-inflammatory drugs. Gut 2003; 52: 1265-1270.
- 40. Hallas J, Lauritsen J, Dalsgard Villadsen H, Freng Gram L. Nonsteroidal anti-inflammatory drugs and upper gastrointestinal bleeding, identifying high-risk groups by excess risk estimates. Scand J Gastroenterol 1995; 30: 438-444.
- 41. MacDonald TM, Morant SV, Robinson GC, et al. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ 1997; 315: 1333-1337.
- 42. Mellemkjaer L, Blot WJ, Toft Sorensen H, et al. Upper gastrointestinal bleeding among users of NSAIDs: a population-based cohort study in Denmark. Br J Clin Pharmacol 2002; 53: 173-181.
- 43. Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol 1995; 141: 539-545.
- 44. Perez Gutthann S, Garcia Rodriguez LA, Raiford DS. Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology 1997; 8: 18-24.
- 45. Garcia Rodriguez LA, Cattaruzzi C, Troncon MG, Agostinis L. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med 1998; 158: 33-39.
- 46. Li Q. Statistical reviewer briefing document for the advisory committee for NDA # 21-042 s-007. 2000. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3677b2_04_stats.pdf (accessed Nov 2002).
- 47. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73: 13-22.
- 48. Cox DR, Oakes D. Analysis of survival data. London: Chapman and Hall, 1984.
- 49. Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med 1991; 115: 787-796.
- 50. Hernandez-Diaz S, Garcia Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000; 160: 2093-2099.
- 51. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 2004; 63: 759-766.
- 52. Freston JW. Rationalizing cyclooxygenase (COX) inhibition for maximal efficacy and minimal adverse events. Am J Med 1999; 107(6A): 78S-88S.
- 53. Lipani JA, Poland M. Clinical update of the relative safety of nabumetone in long-term clinical trials. Inflammopharmacology 1995; 3: 351-361.
- 54. Palmer RH, MacDonald B, DeLapp R. Peptic ulcers with nabumetone: experience in 1677 patients with 4033 years of exposure. Gastroenterology 1998; 114 (4 Suppl): A252.
- 55. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, 2005.
- 56. Fletcher RH, Fletcher SW, Wagner EH. Clinical epidemiology: the essentials. 3rd ed. Baltimore, Md: Williams and Wilkins, 1996.
- 57. Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ 1995; 311: 619-620.





Abstract
Objective: Exposure to non-steroidal anti-inflammatory drugs (NSAIDs) is associated with increased risk of serious gastrointestinal (GI) events compared with non-exposure. We investigated whether that risk is sustained over time.
Data sources: Cochrane Controlled Trials Register (to 2002); MEDLINE, EMBASE, Derwent Drug File and Current Contents (1999–2002); manual searching of reviews (1999–2002).
Study selection: From 479 search results reviewed and 221 articles retrieved, seven studies of patients exposed to prescription non-selective NSAIDs for more than 6 months and reporting time-dependent serious GI event rates were selected for quantitative data synthesis. These were stratified into two groups by study design.
Data extraction: Incidence of GI events and number of patients at specific time points were extracted.
Data synthesis: Meta-regression analyses were performed. Change in risk was evaluated by testing whether the slope of the regression line declined over time. Four randomised controlled trials (RCTs) provided evaluable data from five NSAID arms (aspirin, naproxen, two ibuprofen arms, and diclofenac). When the RCT data were combined, a small significant decline in annualised risk was seen: − 0.005% (95% CI, − 0.008% to − 0.001%) per month. Sensitivity analyses were conducted because there was disparity within the RCT data. The pooled estimate from three cohort studies showed no significant decline in annualised risk over periods up to 2 years: − 0.003% (95% CI, − 0.008% to 0.003%) per month.
Conclusions: Small decreases in risk over time were observed; these were of negligible clinical importance. For patients who need long-term (> 6 months) treatment, precautionary measures should be considered to reduce the net probability of serious GI events over the anticipated treatment duration. The effect of intermittent versus regular daily therapy on long-term risk needs further investigation.