Food allergy is a common allergic manifestation in early childhood.1 There has been a significant increase in public awareness of food allergies, as highlighted in media reports in Australia and overseas. However, some medical practitioners remain sceptical about the role of food allergies in a number of clinical syndromes, such as atopic dermatitis, colic and gastro-oesophageal reflux in infancy, despite an increasing body of evidence that food allergy can contribute to these conditions.2 Our article aims to help general practitioners and other clinicians understand the principles of diagnosis and management of food allergy in childhood, and suggests when to refer patients for specialist opinion.
The prevalence of food allergies appears to be increasing in industrialised countries, although reliable, population-based data are limited. Both prevalence figures and the spectrum of food allergens vary considerably between geographical regions, and are thought to reflect the variation in diet between different cultures.1 However, it has been estimated that up to 6% of children under 3 years of age are affected by food allergies.3 Infants with an atopic first-degree relative are at higher risk of allergy.
Recent studies have tried to confirm anecdotal evidence of an increased incidence of peanut allergy. In a UK study, Grundy et al found an increase in reported peanut allergy from 0.5% to 1.5% in two sequential early childhood cohorts from the same geographic area, surveyed 6 years apart.4
food intolerance (eg, lactose malabsorption);
pharmacological reactions to food components (eg, vasoactive amines);
food poisoning (eg, food-borne bacterial gastroenteritis); and
toxic reactions (eg, to staphylococcal enterotoxin).
It is estimated that about a quarter of the population will have an adverse reaction to food (of which food allergy is just one type) during their lifetime, especially during infancy and early childhood.5
Although, in theory, any food protein may have the ability to sensitise the immune system, more than 90% of IgE-mediated food allergies in children are caused by just eight food items: cows milk, soy, hens egg, peanuts, tree nuts (and seeds), wheat, fish and shellfish. Typically, food allergens are glycoproteins that are relatively resistant to digestion and cooking. A large number of food allergens have now been identified and characterised (eg, β-lactoglobulin in cows milk, ovomucoid [Gal d 1] in egg and Arachis hypogaea allergen 1 [Ara h 1] in peanut). On each of these proteins, specific epitopes (structural components of the antigen molecule) have been mapped that interact with food-specific IgE antibody or T cell receptors. Further characterisation of these epitopes will be essential for developing food vaccines or genetically modified hypoallergenic foods. Epitopes also appear to have a prognostic role in food allergies. Linear epitopes are typically associated with long-term, persistent allergies, whereas conformational (three-dimensional) epitopes may be associated with more transient allergies.6
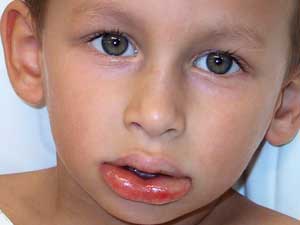
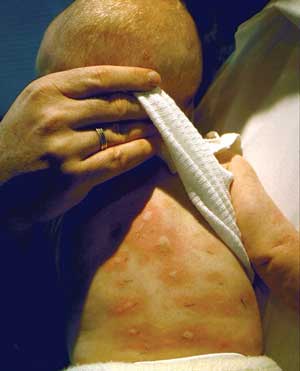
Allergic reactions to foods encompass a spectrum of symptoms, ranging from mild cutaneous involvement to life-threatening anaphylactic reactions (Box 1, Box 2).1 The relationship between food exposure and clinical reaction may be obvious, as in an acute IgE-mediated reaction to peanut ingestion. In such cases, elimination of the food will prevent further symptoms. However, the overall contribution of a food antigen to multifactorial conditions such as atopic dermatitis, eosinophilic oesophagitis, gastro-oesophageal reflux, or infantile colic is less well understood. In these cases, a food protein may induce the disorder or trigger an exacerbation, but elimination of the offending antigen, while reducing the severity of a disease, may not result in complete remission.
Atopic dermatitis in infancy is closely associated with both IgE-mediated and non-IgE-mediated food allergy. The association is strongest in infants with moderate to severe eczema that begins before 12 months of age (Box 3). As food allergy often resolves in early childhood, the association between food allergy and eczema is much weaker in older children and adults than in infants.
Cows milk allergy (CMA) affects about 2% of infants under 2 years of age in industrialised nations and is the most common form of food allergy in this age group. CMA can present with IgE- or non-IgE-mediated manifestations, with up to 50% thought to be non-IgE-mediated. Symptoms and syndromes that should alert the clinician to the possibility of CMA are outlined in Box 1. Importantly, CMA is not limited to formula-fed infants, as intact cows milk proteins (such as β-lactoglobulin and α-lactalbumin) have been found in breastmilk. In most children with CMA, the allergic response develops within 4 weeks of starting cows milk formula,8 and in the great majority of cases CMA resolves by 3 years of age.9
the small intestine: infants with food protein-induced enteropathy present with diarrhoea and failure to thrive;
the colon: the most common cause of low-grade rectal bleeding in young infants is food protein-induced proctocolitis;10,11 and
the small intestine and colon: food protein-induced enterocolitis syndrome (FPIES) is characterised by more extensive disease of the small intestine and colon (see below).12
Other gastrointestinal disorders, such as eosinophilic oesophagitis, have also been shown to be associated with food allergies. This condition is discussed in a separate Focus article in this issue.13
FPIES often presents with profuse diarrhoea, vomiting, dehydration and failure to thrive. In about 20% of patients, the presentation can be dramatic, with acute dehydration leading to episodes of circulatory collapse and shock.12 Allergy to multiple food proteins is common in FPIES, and although cows milk and soy are considered the main causative allergens, infants can present with FPIES following their first exposure to grains (such as oats or wheat), rice or poultry.12 Interestingly, FPIES does not seem to occur in breastfed infants, suggesting that larger amounts of the offending antigen are required to elicit intestinal mucosal inflammation. By contrast, food protein-induced enteropathy and proctocolitis may occur in either formula-fed or breastfed infants.10,11
Multiple food allergy (previously known as “multiple food protein intolerance of infancy”) is characterised by delayed-onset food-allergic reactions to breastmilk, formula milk (including extensively hydrolysed formula [EHF] and soy) and a broad range of solid foods. Infants with multiple food allergy may present with symptoms such as intermittent vomiting, diarrhoea, poor feeding, irritability, severe atopic dermatitis or failure to thrive.14 Resolution of the symptoms occurs only after the introduction of an amino acid-based formula (AAF). Such infants have complex nutritional requirements and should be referred early for specialist assessment and management.
SPT provides a readily available and inexpensive means of assessing IgE-mediated food allergy. There is no minimum age for SPT, which can be performed in babies and infants with useful results. A positive skin prick test result has a relatively low positive predictive value (ie, a significant number of patients with a positive result may be asymptomatic), but a skin prick test has a high negative predictive value (ie, a negative result indicates that IgE-mediated food allergy is unlikely). Non-IgE-mediated mechanisms cannot be assessed by SPT and may require formal food challenges to firmly identify the offending food antigen. Diagnostic SPT decision points have been defined for several food allergens (Box 4).
Food-specific serum IgE antibody levels can be used as an alternative to SPT in assessing IgE-mediated food allergy, although laboratory reference ranges vary widely.20,21 Low levels of food-specific serum IgE may be found in healthy individuals without clinical reactivity to the food (ie, there is sensitisation but not allergy). Food-specific serum IgE levels should be quantified in kUA/L (units are kU/L for total IgE and kUA/L for allergen-specific IgE antibodies) rather than expressed on semi-quantitative scales (such as low/medium/high), as diagnostic decision points are available for several major food allergens (Box 4).
In recent years, the atopy patch test (APT) has been introduced as a diagnostic tool for delayed-onset food allergies, including atopic dermatitis and eosinophilic oesophagitis. The APT is based on cutaneous, cell-mediated responses after epicutaneous application of food allergens. It has been suggested that the APT, in conjunction with IgE-based testing, significantly improves the diagnostic accuracy of allergy testing, again reducing the need for formal challenges.17 However, the role of patch testing in diagnosis of food allergy requires further clarification and is an area of ongoing research.
In patients with equivocal SPT or food-specific IgE results below the diagnostic decision points, confirmation of the diagnosis can only be achieved by formal food challenge.22 Challenge protocols are based on increasing oral doses of food allergen, beginning at a very low dose. The doses are administered at predetermined time intervals until the first symptoms occur. Challenges are usually performed in hospital because of the risk of anaphylaxis. However, home challenges undertaken by parents may be suitable in patients with mild allergic reactions and a negative skin prick test, as the risk of a severe immediate reaction or anaphylaxis is minimal. Open-label challenges are usually sufficient in clinical practice, as long as symptoms can be objectively assessed. Double-blind, placebo-controlled food challenges are used for patients with subjective symptoms or in the research setting.
Elimination diets are also an important diagnostic step in investigating delayed-onset food allergies, which are usually non-IgE-mediated (Box 5). Decisions on whether to undertake formal food challenges and whether to perform them in hospital are influenced by the likelihood of food allergy — based on the history and the interpretation of allergy test results — and the perceived risk of a severe reaction on challenge.
There is not complete agreement on first-line treatment for infants with CMA. About 10% of infants with CMA will not tolerate EHF, presumably because of residual allergenicity of larger peptide and protein molecules present in the formula, and an AAF may be required. The current Australian Pharmaceutical Benefits Scheme (PBS) recommendation is that a soy-based formula should always be trialled before prescribing EHF. However, a recent European position statement has recommended that soy-based formula should not be used as first-line treatment for infants under 6 months of age, because of a high level of concurrent soy allergy and questions about the appropriateness of soy formula in this age group.27 In Europe, EHF is the first-line formula for treating CMA — except in infants suspected of having multiple food allergy, who require an AAF. (Most infants will tolerate several non-formula foods by 18 months and can cease AAF by 3 years of age.28) In the light of international recommendations, the current Australian PBS recommendation may need to be reviewed.
Fact or fiction — true or false?
2. Food allergy does not occur in exclusively breastfed infants (T/F)
Over 90% of fatal or near-fatal anaphylactic reactions to foods are caused by peanuts and tree nuts.29 Intramuscular injection of adrenaline is the treatment of choice for anaphylaxis, regardless of aetiology. EpiPen auto-injectors (CSL Limited, Melbourne, VIC) containing a single dose of adrenaline are available for emergency treatment of anaphylaxis. The doses commonly recommended by specialist bodies (such as the Australasian Society of Clinical Immunology and Allergy [ASCIA]) differ from those in the manufacturer’s product information. In Australia, it is recommended that EpiPen Junior (0.15 mg) be prescribed for patients weighing 10–20 kg and EpiPen (0.30 mg) for patients over 20 kg.
The use of adrenaline auto-injectors in Australian children has increased by 300% over the past 5 years, with 1 in 544 Australian children aged under 10 years now using them.30 This may indicate that EpiPen is being prescribed to patients in low-risk categories, but no population-based data are currently available on the appropriateness of EpiPen use in Australia.
Guidelines published by ASCIA recommend that patients with previous food-induced anaphylaxis should be provided with an EpiPen.31 Its prescription should also be considered for patients with a history of a significant generalised allergic reaction and at least one of the following risk factors: age over 5 years, history of asthma, allergy to peanuts or tree nuts, or limited access to emergency medical care.
One hypothesis to explain the increased incidence of sensitisation to food allergens is that the reduction in early childhood infections or in exposure to microbial products (eg, endotoxin) may impede the development of early immunoregulatory responses. This leaves the immune system more susceptible to inappropriate reactivity to innocuous antigens, resulting in an “allergic” reaction.32
Postnatal development of mucosal immune homoeostasis is influenced by the type of commensal microbiota present in the neonatal period (eg, the predominance of bifidobacteria in breastfed infants may be protective against food allergy), as well as the initial timing and dose of dietary antigens.33 Recent research suggests that toll-like receptor-dependent signals provided by intestinal bacteria may inhibit the development of allergic responses to food antigens via stimulation of regulatory T cells.34
A recent study found that differences in the neonatal gut microbiota precede the development of atopy, suggesting a role for commensal intestinal bacteria in the prevention of allergy.35 This research has led to the hypothesis that probiotics may promote oral tolerance. Perinatal administration of Lactobacillus casei GG has been reported to reduce the incidence of atopic dermatitis, but not food allergy, in at-risk children during the first 4 years of life.
Exclusive breastfeeding seems to have a preventive effect on the early development of asthma and atopic dermatitis up to 2 years of age, but the evidence for prevention of food allergies is less clear. The delayed introduction of solids until after 4 months is believed to partially protect infants from developing food allergies, but this has recently been questioned.36 If exclusive breastfeeding is not possible, a hydrolysed formula is recommended for the first 4 months of life in infants at high risk of food allergy (ie, those with an atopic first-degree relative).37 Currently there is no evidence for the protective role of maternal elimination diets during pregnancy.25
Several novel treatments for food allergy are currently under evaluation. However, none of these are currently available outside clinical trials. The role of injectable immunotherapy38 for treating food allergy is limited because of the high risk of inducing anaphylaxis. By contrast, sublingual immunotherapy to food allergens may be better tolerated in children, although its clinical efficacy has not yet been clearly shown.
Recombinant anti-IgE antibody (omalizumab) has been used with limited success to treat food allergy. A recent study of patients with severe peanut allergy showed an increased threshold of tolerance (on average, from one-half to nine peanuts) on oral food challenge after being given a course of omalizumab.39 Although such a protocol might protect an individual with severe peanut allergy against most inadvertent peanut ingestions, the therapy is expensive, requires regular administration, and is not currently approved in Australia for the treatment of food allergy.
Finally, there is the prospect of producing genetically modified foods from which the major allergens have been removed.40
1 Spectrum of allergic reactions to food proteins
2 Symptoms that should alert the clinician to the possibility of food allergy in children, particularly in the first months of life*
Clear relationship between food and symptoms (high risk)
Anaphylaxis, generalised allergic reaction (angioedema, erythema, urticaria) or severe vomiting within 1–2 hours of ingesting a newly introduced food
Oral allergy syndrome (oral/perioral pruritus associated with food-specific serum IgE antibodies)
Food protein-induced eosinophilic gastrointestinal syndromes of infancy (persistent vomiting or bloody diarrhoea in first months of life)
Gastro-oesophageal reflux, unresponsive to acid suppression
Atopic dermatitis presenting in the first 12 months of life, unresponsive to topical treatment
Severe unremitting infantile colic presenting in the first weeks of life
Persistent constipation in infancy, with onset at the introduction of cows milk formula
* Modified from an American Gastroenterological Association position statement.7
4 Diagnostic decision points
SPT diagnostic decision points have been defined for several food allergens, including cows milk, egg and peanut.15,16 These are cut-off values for SPT weal diameters that predict a positive food challenge result with over 95% accuracy. Correlation of a clear clinical reaction to a food antigen with a skin prick test result above a diagnostic decision point has reduced the need for formal food challenges. Predictive SPT weal diameters have been shown to be smaller in young children under 2 years of age.15
Diagnostic decision points for food-specific IgE levels have been defined for cows milk, egg, peanut and fish. These are the cut-off food-specific IgE levels that predict positive food challenges with at least 95% accuracy.17 The decision points for cows milk- and egg-specific serum IgE in infants under 2 years of age are lower than in older patients.18,19
5 Evidence-based practice tips*
Elimination diets are an important step in the diagnosis of delayed-onset food allergies, as these are usually non-IgE-mediated (Level III-2).23
Children with moderate to severe atopic dermatitis not responding to topical steroids and presenting in the first 6 months of life should be assessed for food allergies (Level III-2).24
Mothers with a family history of atopy need not undertake an elimination diet to specific foods during pregnancy in order to prevent food allergy (Level I).25
*Based on National Health and Medical Research Council levels of evidence.26
- Katrina J Allen1,2,3
- David J Hill2
- Ralf G Heine1,2,3
- 1 Department of Allergy and Immunology, Royal Children’s Hospital, Melbourne, VIC.
- 2 Murdoch Children’s Research Institute, Royal Children’s Hospital, Melbourne, VIC.
- 3 Department of Paediatrics, University of Melbourne, Melbourne, VIC.
David Hill has received support for clinical research projects from SHS/Nutricia and presented lectures at sponsored meetings.
- 1. Hill DJ, Hosking CS, Heine RG. Clinical spectrum of food allergy in children in Australia and South-East Asia: identification and targets for treatment. Ann Med 1999; 31: 272-281.
- 2. Heine RG. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Curr Opin Allergy Clin Immunol 2006; 6: 220-225.
- 3. Sampson HA. 9. Food allergy. J Allergy Clin Immunol 2003; 111 (2 Suppl): S540-S547.
- 4. Grundy J, Matthews S, Bateman B, et al. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol 2002; 110: 784-789.
- 5. Schafer T, Bohler E, Ruhdorfer S, et al. Epidemiology of food allergy/food intolerance in adults: associations with other manifestations of atopy. Allergy 2001; 56: 1172-1179.
- 6. Vila L, Beyer K, Jarvinen KM, et al. Role of conformational and linear epitopes in the achievement of tolerance in cow’s milk allergy. Clin Exp Allergy 2001; 31: 1599-1606.
- 7. American Gastroenterological Association medical position statement: guidelines for the evaluation of food allergies. Gastroenterology 2001; 120: 1023-1025.
- 8. Host A, Halken S, Jacobsen HP, et al. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 2002; 13 Suppl 15: 23-28.
- 9. Saarinen KM, Pelkonen AS, Makela MJ, Savilahti E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol 2005; 116: 869-875.
- 10. Chang JW, Wu TC, Wang KS, et al. Colon mucosal pathology in infants under three months of age with diarrhea disorders. J Pediatr Gastroenterol Nutr 2002; 35: 387-390.
- 11. Lake AM. Dietary protein enterocolitis. Curr Allergy Rep 2001; 1: 76-79.
- 12. Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics 2003; 111 (4 Pt 1): 829-835.
- 13. Kakakios A, Heine RG. Eosinophilic oesophagitis. Med J Aust 2006; 185: 401. <eMJA full text>
- 14. Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. J Pediatr Gastroenterol Nutr 2000; 30 Suppl: S67-S76.
- 15. Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy 2000; 30: 1540-1546.
- 16. Verstege A, Mehl A, Rolinck-Werninghaus C, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy 2005; 35: 1220-1226.
- 17. Roehr CC, Reibel S, Ziegert M, et al. Atopy patch tests, together with determination of specific IgE levels, reduce the need for oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol 2001; 107: 548-553.
- 18. Garcia-Ara C, Boyano-Martinez T, Diaz-Pena JM, et al. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J Allergy Clin Immunol 2001; 107: 185-190.
- 19. Boyano-Martinez T, Garcia-Ara C, Diaz-Pena JM, et al. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy 2001; 31: 1464-1469.
- 20. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001; 107: 891-896.
- 21. Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy 2005; 35: 268-273.
- 22. Bock SA, Sampson HA, Atkins FM, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol 1988; 82: 986-997.
- 23. Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology 2005; 128: 1089-1113.
- 24. Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. J Pediatr 2000; 137: 475-479.
- 25. Prescott SL, Tang ML. The Australasian Society of Clinical Immunology and Allergy position statement: summary of allergy prevention in children. Med J Aust 2005; 182: 464-467. <MJA full text>
- 26. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Canberra: NHMRC, 2000. http://www.nhmrc.gov.au/publications/_files/cp69.pdf (accessed Jul 2006).
- 27. ESPGHAN Committee on Nutrition; Agostoni C, Axelsson I, Goulet O, et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2006; 42: 352-361.
- 28. Hill DJ, Heine RG, Cameron DJ, et al. The natural history of intolerance to soy and extensively hydrolyzed formula in infants with multiple food protein intolerance. J Pediatr 1999; 135: 118-121.
- 29. Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 2001; 107: 191-193.
- 30. Kemp AS. EpiPen epidemic: suggestions for rational prescribing in childhood food allergy. J Paediatr Child Health 2003; 39: 372-375.
- 31. Baumgart K, Brown S, Gold M, et al; Australasian Society of Clinical Immunology and Allergy Anaphylaxis Working Party. ASCIA guidelines for prevention of food anaphylactic reactions in schools, preschools and child-care centres. J Paediatr Child Health 2004; 40: 669-671.
- 32. Bailey M, Haverson K, Inman C, et al. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proc Nutr Soc 2005; 64: 451-457.
- 33. Brandtzaeg PE. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann N Y Acad Sci 2002; 964: 13-45.
- 34. Bashir MEH, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004; 172: 6978-6987.
- 35. Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107: 129-134.
- 36. Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics 2006; 117: 401-411.
- 37. Halken S, Host A. Prevention. Curr Opin Allergy Clin Immunol 2001; 1: 229-236.
- 38. Weiner JM. Allergen injection immunotherapy. Med J Aust 2006; 185: 234. <MJA full text>
- 39. Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348: 986-993.
- 40. Lehrer SB. Genetic modification of food allergens. Ann Allergy Asthma Immunol 2004; 93 (5 Suppl 3): S19-S25.





Abstract
Food allergies in children present with a wide spectrum of clinical manifestations, including anaphylaxis, urticaria, angioedema, atopic dermatitis and gastrointestinal symptoms (such as vomiting, diarrhoea and failure to thrive).
Symptoms usually begin in the first 2 years of life, often after the first known exposure to the food.
Immediate reactions (occurring between several minutes and 2 hours after ingestion) are likely to be IgE-mediated and can usually be detected by skin prick testing (SPT) or measuring food-specific serum IgE antibody levels.
Over 90% of IgE-mediated food allergies in childhood are caused by eight foods: cows milk, hens egg, soy, peanuts, tree nuts (and seeds), wheat, fish and shellfish. Anaphylaxis is a severe and potentially life-threatening form of IgE-mediated food allergy that requires prescription of self-injectable adrenaline.
Delayed-onset reactions (occurring within several hours to days after ingestion) are often difficult to diagnose. They are usually SPT negative, and elimination or challenge protocols are required to make a definitive diagnosis. These forms of food allergy are not usually associated with anaphylaxis.
The mainstay of diagnosis and management of food allergies is correct identification and avoidance of the offending antigen.
Children often develop tolerance to cows milk, egg, soy and wheat by school age, whereas allergies to nuts and shellfish are more likely to be lifelong.