Anaphylaxis is a severe immediate-type generalised hypersensitivity reaction affecting multiple organ systems and characterised at its most severe by bronchospasm, upper airway angioedema and/or hypotension. It has also been defined simply as “a serious allergic reaction that is rapid in onset and may cause death”.1 This review aims to help general practitioners and emergency physicians with their approach to acute management and follow-up care in cases of anaphylaxis.
Anaphylaxis is uncommon but not rare, with new cases arising at rates of between 8.4 and 21 per 100 000 patient-years.2-4 An Australian survey of parent-reported allergy and anaphylaxis found that 1 in 170 school children had suffered at least one episode of anaphylaxis.5 Another Australian study showed that, in areas where native Myrmecia ant species are prevalent, 1 in 50 adults have experienced anaphylaxis after stings from native Myrmecia species (Box 1) or honeybees.6 Deaths from anaphylaxis are uncommon, estimated to occur at a rate of 1 per 3 million population per year.7 In areas where sting allergy is common, the death rate may be higher than this. Hospital-based studies suggest a death rate in the order of 1 per 100–200 episodes of anaphylaxis treated in an emergency department.8,9
There is some evidence that the incidence of food allergy and anaphylaxis — like that of allergic rhinitis and atopic dermatitis — may be increasing.10-12
Food, insect venoms or medication trigger most cases of anaphylaxis, with a variable proportion of patients experiencing idiopathic anaphylaxis (in which extensive evaluation fails to identify an underlying cause) (Box 2). In emergency department studies, food allergy is the commonest cause in children — responsible for about 80% of anaphylactic reactions in which the cause has been identified13 — whereas, in adults, foods are implicated in only 20%–30% of cases.8,9 This difference is reflected in mortality statistics: the median ages for lethal reactions to foods and to insect venoms or medications are 22–24 years and 55–67 years, respectively.14
Cofactors are sometimes required before an allergen will provoke a reaction. Factors associated with increased risk of anaphylaxis include intercurrent infection, concomitant medication/foods (particularly α-blockers, β-blockers, angiotensin-converting enzyme [ACE] inhibitors, non-steroidal anti-inflammatory drugs [NSAIDS], alcohol or spicy food), high ambient temperatures and exercise. So-called “summation anaphylaxis” may explain intermittent anaphylaxis despite frequent allergen exposure, and may account for some cases in which a cause has not been established.15 One of the most common cofactors, predominantly affecting young adults, is physical exercise. Some experience symptoms with exercise alone; others do so only if allergenic foods (most commonly wheat, celery, seafood, nuts, fruit or vegetables) are ingested within a few hours prior to exercise.
Mast cell activation results in the release of many mediators that include histamine, leukotrienes, tumour necrosis factor and various cytokines. The large numbers of mediators provide redundancy and positive feedback mechanisms whereby other effector cells are recruited to release more mediators, perpetuating the allergic response. This amplification and perpetuation, which has been referred to as a “mast cell leukocyte cytokine cascade”,16 underscores the importance of physiological antagonism with adrenaline and fluid resuscitation, rather than antagonism of a single mediator such as histamine.
Anaphylactic mediators cause vasodilation, fluid extravasation, smooth muscle contraction and increased mucosal secretions. Death may occur from hypoxaemia (due to upper airway angioedema, bronchospasm and mucus plugging) and/or shock (due to massive vasodilation, fluid shift into the extravascular space and depressed myocardial function).17 While compensatory tachycardia in response to hypotension is considered a characteristic feature, sudden bradycardia with cardiovascular collapse and cardiac arrest may occur before any skin features become apparent.18 The cause of this phenomenon is unclear, but it is an important clinical feature to recognise in order to avoid making an initial misdiagnosis of a “panic attack” or “vasovagal reaction” in cases where dyspnoea, nausea, anxiety, and bradycardia may occur just before cardiovascular collapse.
The clinical features of anaphylaxis are summarised in Box 3. Skin features are almost universal if reactions are closely observed, but erythema (and even angioedema) may be subtle and missed if not carefully looked for (Box 4). Respiratory symptoms are more common in children, whereas cardiovascular and cutaneous symptoms dominate in adults.13 In part, this may be related to the higher frequency of atopy, asthma and food allergy in children.13 Pre-existing lung disease is associated with an increased frequency of respiratory compromise from any cause,6,9 and poorly controlled asthma appears to be the main risk factor for childhood death due to food allergy.19
Confusion, collapse, unconsciousness and incontinence are strongly associated with the presence of hypotension and hypoxia. In adults, the occurrence of dyspnoea, profuse sweating, nausea, vomiting and abdominal pain are also significant, as they correlate with the presence of hypotension.9
Anaphylaxis is a rapidly evolving generalised multi-system allergic reaction characterised by one or more symptoms or signs of respiratory and/or cardiovascular involvement and involvement of other systems such as the skin and/or the gastrointestinal tract.20
This definition will exclude some atypical yet still life-threatening reactions, and more recently, an international consensus working definition has been proposed to address these issues.1 However, no definition has yet been subject to prospective validation. Thus it may be important at times to initiate treatment for a suspected case of anaphylaxis with cardiovascular or respiratory compromise, even if consensus diagnostic criteria have not been met.
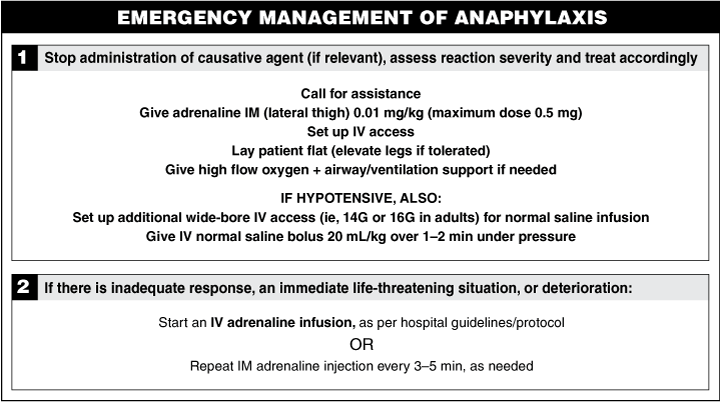
Acute management of anaphylaxis (Box 6, Box 7) includes the following:
Place the patient in the supine position (or left lateral position for vomiting patients);
Give intramuscular adrenaline;
Resuscitate with intravenous saline (20 mL/kg body weight, repeated up to a total of 50 mL/kg over the first half hour);
Support the airway and ventilation; and
Give supplementary oxygen.17
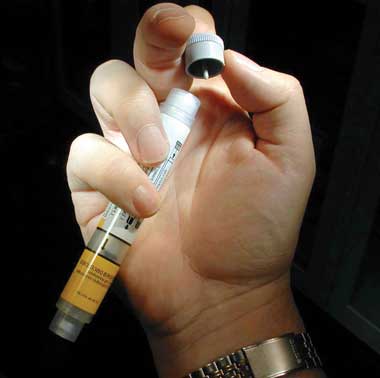
Intramuscular 1 : 1000 (1 mg/mL) adrenaline at a dose of 0.01 mg/kg (0.01 mL/kg) body weight up to a maximum dose of 0.5 mg (0.5 mL) injected into the lateral thigh (vastus lateralis) has the advantage that it can be given without delay, is absorbed more reliably than injections into other locations or subcutaneously,21,22 is anecdotally effective in most cases when given early, and avoids the potentially lethal effects of large intravenous bolus injections.14 The appropriate dose of EpiPen (CSL Limited, Melbourne, VIC) (Box 8) can be used instead, if available. The intramuscular dose can be repeated after 3–5 minutes if required.
If resuscitation using intramuscular adrenaline and volume expansion with intravenous saline is ineffective, an infusion of intravenous adrenaline may be required, but this should be done only by experienced hands. Intravenous boluses of adrenaline are potentially dangerous and should not be used unless cardiac arrest is imminent. Controlled intravenous infusions of adrenaline were shown to be safe and effective in one prospective Australian study.18 An infusion protocol derived from this study has been published elsewhere.17
Persistent bronchospasm may respond to treatment with additional bronchodilators. If intubation is required, continuous puffs of salbutamol through an aerosol port directly into the ventilation circuit may help to “break” severe bronchospasm.
Persistent stridor may respond to continuous nebulised adrenaline (5 mg in 5 mL [ie, five 1 mg ampoules]) in addition to parenteral adrenaline. Surgical airway intervention (cricothyrotomy) may be required.
Persistent hypotension may be due to either profound vasodilation or cardiac failure. Anecdotally, vasodilation may respond to vasopressors such as metaraminol or vasopressin.26-28 In patients who have pre-existing heart failure or are taking β-blockers, a phosphodiesterase inhibitor such as glucagon may be tried.29,30
Medications such as antihistamines, H2 receptor antagonists, corticosteroids and antileukotrienes have no proven impact on the immediate and dangerous effects of anaphylaxis, although they may ameliorate mild allergic reactions confined to the skin. The only registered antihistamine for parenteral use in Australia, promethazine, can worsen vasodilation and hypotension, and its use is not advised.17,31 Until human research clarifies the potential risks and benefits of antihistamines, it is prudent to restrict antihistamine use to oral, selective, non-drowsiness-inducing antihistamines, with or without oral or injectable corticosteroids, for the symptomatic relief of mild skin symptoms. Based on their use in treating asthma, corticosteroids are commonly given to reduce the risk of biphasic anaphylaxis (see below), although there is currently no evidence to support their effectiveness for this purpose.
Anaphylaxis remains a largely clinical diagnosis. Serum mast cell tryptase concentration can be determined, but this is an insensitive biomarker for anaphylaxis, although serial measurements (eg, on arrival, 1 hour later and before discharge) may improve sensitivity and specificity.32 An elevated tryptase level may be a useful clue when the diagnosis is uncertain, but a normal result does not exclude anaphylaxis.
The time course of anaphylaxis can be classified as “uniphasic”, “protracted” or “biphasic”.33 Although most reactions respond rapidly to treatment and do not recur (uniphasic reactions), an observation period is recommended. This is because, in some patients, symptoms may fail to improve or may worsen as the effect of adrenaline wears off (protracted anaphylaxis) or may return after early resolution (biphasic reaction). No clinical feature consistently identifies patients at risk of a biphasic reaction. Expert consensus is that a reasonable length of observation after symptom resolution is 4–6 hours in most patients, with more prolonged observation in those with severe or refractory symptoms and those with reactive airway disease, as most fatalities associated with anaphylaxis occur in these patients.1
Before examining the surrounding circumstances to define a cause for the patient’s symptoms (Box 2), it is important to first determine whether anaphylaxis occurred by carefully reviewing the available documentation. Short-lived bouts of urticaria and/or angioedema lasting less than 12 hours should prompt suspicion of an allergic cause, although on their own they do not satisfy a definition of anaphylaxis. As typical cutaneous features may be absent in up to 20% of cases, anaphylaxis should be considered in the differential diagnosis of any episode of severe, acute-onset respiratory distress, bronchospasm or cardiovascular collapse (Box 9).
Details of exposure to potential triggers, including occupational allergens (eg, latex) and cofactors, in the preceding 8 hours should be recorded while memory of the event is fresh. Almost all anaphylactic reactions to insect venoms or to food or medication occur within 1 and 6 hours, respectively.2
Medical practitioners should record the presence of known food or drug hypersensitivity and consider the possibility of accidental exposure. Ask patients about symptoms of contact urticaria (eg, during food preparation) or itching in the mouth and throat after eating certain foods (oral allergy syndrome). The latter indicates an allergy to structurally similar proteins in pollen and in some fruit and vegetables.2 If an insect sting has occurred, factors that may help identify the cause are the insect’s appearance, the presence of a stinger left in the skin (pathognomonic for honeybee sting) and the location where the sting occurred (stings by jack jumper ants or bulldog ants are more common in bushland).
Anaphylaxis to insect stings can be prevented with venom immunotherapy,23 which reduces the risk of anaphylaxis from repeated stings and is associated with an improved quality of life compared with carrying an EpiPen alone.24 Attempts to modify the severity of food allergy using similar techniques have thus far failed, although novel methods of inducing tolerance hold some promise for the future.35
For most patients, anaphylaxis is a disorder for which the risk of relapse is chronic but the event itself is unpredictable.2 The mainstays of long-term management of anaphylaxis include:
Specialist assessment.
Identification and avoidance of triggers and cofactors, if possible. Common triggers of anaphylaxis include food, stinging insects and medication. Exercise, alcohol consumption and taking NSAIDS are common cofactors.
Avoidance of medications that may complicate management.
Training patients to recognise early warning symptoms and to carry self-injectable adrenaline (EpiPen) (after being trained in its use).
Provision of a written anaphylaxis action plan.
Identification of at-risk patients with a MedicAlert bracelet and entry of an allergy alert into hospital or health care network clinical information systems.
A number of resources for patients and health care professionals, including guidelines for dealing with anaphylaxis in specific settings such as schools and childcare centres, prescribing guidelines and written action plans, are available on the ASCIA website (http://www.allergy.org.au/anaphylaxis). The EpiPen doses commonly recommended by specialist bodies (such as ASCIA) differ from those in the package insert. ASCIA recommends prescribing EpiPen Junior (0.15 mg) for patients weighing 10–20 kg and EpiPen (0.3 mg) for patients weighing over 20 kg.
Despite their widespread clinical use, medications such as antihistamines and corticosteroids have no proven efficacy in preventing or treating anything other than mild cutaneous allergic symptoms. Furthermore, the cost of maintaining or using these medications in people with anaphylaxis due to other causes needs to be balanced against the cost of purchasing an additional EpiPen. Large doses of adrenaline may be required to treat hypotensive anaphylaxis,18 so Australians living in remote areas may need additional supplies beyond the single EpiPen unit subsidised by the current Pharmaceutical Benefits Scheme for individuals over 17 years of age. Even if an initial EpiPen injection has been effective, patients should seek emergency medical care without delay. Patients who live or work in remote areas should consider having access to means of summoning emergency assistance, such as a mobile telephone or, in some cases, an emergency satellite beacon.
Despite the frightening nature of anaphylactic episodes, compliance with advice to avoid known triggers and to carry and use injectable adrenaline is nowhere near 100%.2 Denial and “acting out” by teenagers is also common, and peer pressure or bullying at school may prompt some patients to take unnecessary risks. Review from time to time or after further episodes offers an opportunity to re-educate patients on the use of EpiPen and to ensure that the device is renewed at appropriate intervals. Psychological morbidity and negative impact on quality of life are not uncommon in patients and their caregivers, and some require emotional support and counselling as well as medical advice.
2 Causes of anaphylaxis
Insect stings: most commonly honeybee, Australian native ants, wasps
Foods: most commonly peanuts, tree nuts, egg, seafood, cows milk, dairy products, seeds
Medications: most commonly antibiotics, non-steroidal anti-inflammatory drugs
Unidentified (no cause found)
Physical triggers (eg, exercise, cold)
Biological fluids (eg, transfusions, immunoglobulin, antivenoms, semen)
Latex
Tick bites
Hormonal changes: breastfeeding, menstrual factors
Dialysis membranes (haemodialysis-associated anaphylaxis)
Hydatid cyst rupture
Aeroallergens: domestic/laboratory animals, pollen
Food additives: monosodium glutamate, metabisulfite, preservatives, colours, natural food chemicals
Topical medications (eg, antiseptics)
3 Clinical features of anaphylaxis
5 Differential diagnosis of anaphylaxis
Idiopathic urticaria
Isolated angioedema*
Idiopathic
ACE inhibitor-induced
Acquired or hereditary C1 esterase inhibitor deficiency
Conditions mimicking upper airway oedema
Dystonic reactions mimicking symptoms of a swollen tongue after taking metoclopramide, prochlorperazine or antihistamines
Acute oesophageal reflux (sudden onset of painful throat “swelling”)
Peptide-secreting tumours (eg, carcinoid syndrome, VIPomas†)
Alcohol-related
Medullary carcinoma of thyroid
“Red man syndrome”‡
Vasovagal episodes
Systemic inflammatory response syndrome
Shock (septic, cardiogenic, haemorrhagic)
7 Evidence-based practice tips*
Recommended acute management of a patient with anaphylaxis is to place the patient in a supine position and give adrenaline and intravenous volume resuscitation (Level IV).1
Intramuscular injection into the lateral thigh (vastus lateralis) is preferred to injections into arm or deltoid muscles or subcutaneously, because of better absorption (Level III-1).21,22
A controlled intravenous infusion of adrenaline is a safe and effective management for anaphylaxis (Level III-3).18
A reasonable length of observation after symptom resolution is 4–6 hours in most patients, with more prolonged observation recommended in patients with severe or refractory symptoms (Level IV).1
Venom immunotherapy prevents anaphylaxis to insect stings and significantly improves quality of life compared with carrying injectable adrenaline (EpiPen) alone (Level II).23,24
* Based on National Health and Medical Research Council levels of evidence.25
- Simon G A Brown1
- Raymond J Mullins2
- Michael S Gold3
- 1 University of Western Australia and Fremantle Hospital, Fremantle, WA.
- 2 Australian National University, Canberra, ACT.
- 3 Women’s and Children’s Hospital and University of Adelaide, Adelaide, SA.
The authors are grateful for the many helpful comments by Dr Bob Heddle.
None identified.
- 1. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report — second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006; 117: 391-397.
- 2. Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy 2003; 33: 1033-1040.
- 3. Peng MM, Jick H. A population-based study of the incidence, cause, and severity of anaphylaxis in the United Kingdom. Arch Intern Med 2004; 164: 317-319.
- 4. Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J Allergy Clin Immunol 1999; 104: 452-456.
- 5. Boros CA, Kay D, Gold MS. Parent reported allergy and anaphylaxis in 4173 South Australian children. J Paediatr Child Health 2000; 36: 36-40.
- 6. Brown SGA, Franks RW, Baldo BA, Heddle RJ. Prevalence, severity, and natural history of jack jumper ant venom allergy in Tasmania. J Allergy Clin Immunol 2003; 111: 187-192.
- 7. Moneret-Vautrin DA, Morisset M, Flabbee J, et al. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy 2005; 60: 443-451.
- 8. Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol 2001; 108: 861-866.
- 9. Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004; 114: 371-376.
- 10. Robertson CF, Roberts MF, Kappers JH. Asthma prevalence in Melbourne schoolchildren: have we reached the peak? Med J Aust 2004; 180: 273-276. <MJA full text>
- 11. Sheikh A, Alves B. Hospital admissions for acute anaphylaxis: time trend study. BMJ 2000; 320: 1441.
- 12. Sly RM. Changing prevalence of allergic rhinitis and asthma. Ann Allergy Asthma Immunol 1999; 82: 233-248; quiz 248-252.
- 13. Braganza SC, Acworth JP, McKinnon DR, et al. Paediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child 2006; 91: 159-163.
- 14. Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000; 30: 1144-1150.
- 15. Ring J, Brockow K, Behrendt H. History and classification of anaphylaxis. Novartis Found Symp 2004; 257: 6-16; discussion 16-24, 45-50, 276-285.
- 16. Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol 2000; 105: 847-859.
- 17. Brown SGA. Anaphylaxis: clinical concepts and research priorities. Emerg Med Australas 2006; 18: 155-169.
- 18. Brown SGA, Blackman KE, Stenlake V, Heddle RJ. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J 2004; 21: 149-154.
- 19. Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol 2004; 4: 285-290.
- 20. Australasian Society of Clinical Immunology and Allergy Anaphylaxis Working Party. Guidelines for EpiPen prescription. Sydney: ASCIA, 2004. http://www.allergy.org.au/anaphylaxis/epipen_guidelines.htm (accessed Feb 2006).
- 21. Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108: 871-873.
- 22. Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101 (1 Pt 1): 33-37.
- 23. Brown SGA, Wiese MD, Blackman KE, Heddle RJ. Ant venom immunotherapy: a double-blind, placebo-controlled, crossover trial. Lancet 2003; 361: 1001-1006.
- 24. Oude Elberink JN, De Monchy JG, Van Der Heide S, et al. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol 2002; 110: 174-182.
- 25. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Canberra: NHMRC, 2000. http://www.nhmrc.gov.au/publications/_files/cp69.pdf (accessed Jul 2006).
- 26. Heytman M, Rainbird A. Use of alpha-agonists for management of anaphylaxis occurring under anaesthesia: case studies and review. Anaesthesia 2004; 59: 1210-1215.
- 27. Schummer W, Schummer C, Wippermann J, Fuchs J. Anaphylactic shock: is vasopressin the drug of choice? Anesthesiology 2004; 101: 1025-1027.
- 28. Kill C, Wranze E, Wulf H. Successful treatment of severe anaphylactic shock with vasopressin. Two case reports. Int Arch Allergy Immunol 2004; 134: 260-261.
- 29. Zaloga GP, DeLacey W, Holmboe E, Chernow B. Glucagon reversal of hypotension in a case of anaphylactoid shock. Ann Intern Med 1986; 105: 65-66.
- 30. Thomas M, Crawford I. Best evidence topic report. Glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J 2005; 22: 272-273.
- 31. Brown SGA. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol 2005; 5: 359-364.
- 32. Brown SGA, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis? Emerg Med Australas 2004; 16: 120-124.
- 33. Stark BJ, Sullivan TJ. Biphasic and protracted anaphylaxis. J Allergy Clin Immunol 1986; 78 (1 Pt 1): 76-83.
- 34. Mullins RJ, Heddle RJ, Smith P. Non-conventional approaches to allergy testing: reconciling patient autonomy with medical practitioners’ concerns [editorial]. Med J Aust 2005; 183: 173-174. <MJA full text>
- 35. Leung DY, Sampson HA, Yunginger JW, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348: 986-993.







Abstract
Anaphylaxis is a serious, rapid-onset, allergic reaction that may cause death. Severe anaphylaxis is characterised by life-threatening upper airway obstruction, bronchospasm and/or hypotension.
Anaphylaxis in children is most often caused by food. Bronchospasm is a common symptom, and there is usually a background of atopy and asthma.
Venom- and drug-induced anaphylaxis are more common in adults, in whom hypotension is more likely to occur.
Diagnosis can be difficult, with skin features being absent in up to 20% of people. Anaphylaxis must be considered as a differential diagnosis for any acute-onset respiratory distress, bronchospasm, hypotension or cardiac arrest.
The cornerstones of initial management are putting the patient in the supine position, administering intramuscular adrenaline into the lateral thigh, resuscitation with intravenous fluid, support of the airway and ventilation, and giving supplementary oxygen.
If the response to initial management is inadequate, intravenous infusion of adrenaline should be commenced. Use of vasopressors should be considered if hypotension persists.
The patient should be observed for at least 4 hours after symptom resolution and referred to an allergist to assist with diagnosis, allergen avoidance measures, risk assessment, preparation of an action plan and education on the use of self-injectable adrenaline. Provision of a MedicAlert bracelet should also be arranged.