Age-related macular degeneration (AMD) is the leading cause of severe visual loss in our community, and is responsible for nearly half the cases of legal blindness in Australia.1 It is a progressive, late-onset degenerative disease affecting central vision, which is essential for activities such as driving and reading. The resultant severe disability involves enormous personal costs and a massive burden on health resources. AMD is generally regarded as a complex genetic disorder with the added impact of environmental risk factors. To date, the pathogenesis of AMD remains unknown.
The prevalence of AMD increases exponentially with age (Box).1 Australian data from the Visual Impairment Project and the Blue Mountains Eye Study predict that the number of people in Australia with AMD will double in the next 20 years, with direct costs to the community reaching over $1 billion by 2020.2 Unfortunately, current treatment options for AMD have limited success, with even the newest treatments, such as photodynamic therapy and vascular endothelial growth factor (VEGF) inhibitors, applicable to only some cases, and lost vision is seldom regained.
Observational studies have revealed that AMD shares a number of overlapping risk factors with atherosclerosis, among which age1 and cigarette smoking1,3 have been most consistently demonstrated. A dose–response relationship has also been found between smoking and neovascular AMD.4 A recent review confirmed that smoking was the most important and only agreed upon modifiable risk factor for AMD.5
Observational studies linking AMD to systemic hypertension have given conflicting results. A cohort study of 1828 subjects found that a longer duration of systemic hypertension was associated with a higher prevalence of early AMD.6 Another large cross-sectional study found that AMD was associated with increased systolic blood pressure,7 while a smaller study linked it to diastolic blood pressure.8 A large case–control study by the Age Related Eye Disease Study group found a positive association between diastolic blood pressure, history of hypertension and use of antihypertensive medication, and wet AMD but not dry AMD.9 However, several recent, large observational studies with over 4000 subjects failed to confirm any significant link between AMD and hypertension.10,11
There is also some evidence from observational studies linking AMD to body mass index. Results from several large studies have all shown a positive relationship between early AMD and high body mass index.11-13 Waist-to-hip ratio has also been implicated in development of early AMD in women.13
With regard to actual associations of AMD with atherosclerosis, the data have been inconsistent. Some observational studies have shown positive associations between AMD and atherosclerosis,14,15 while others found a positive association between AMD and carotid artery atherosclerotic plaques.15 Other large observational studies have found no such association.10,11,16
If the association between cardiovascular risk factors, cardiovascular disease (CVD) and AMD holds, then treatment of CVD itself could have an impact on AMD. Based on this rationale and theories about the possible benefits of the cholesterol-lowering statin family of drugs, the relationship between AMD and the use of statins has been examined in several clinical studies, with contradictory results.17 However, these studies have been either cross-sectional or cohort studies relying largely on patient recall of the medications taken, and there have been no randomised controlled trials (RCTs) of statins in patients with AMD published to date. Thus, it is not clear whether statins have a protective effect in AMD.18,19 An RCT using simvastatin (Merck Sharp & Dohme) is currently under way in Australia.20
Evidence of a possible role of fatty acids in AMD has come from several sources: links between cardiovascular disease and AMD; the association of a cholesterol pathway gene, the apolipoprotein E gene, with AMD; and the fact that the lipid content of the Bruch membrane — a semipermeable membrane that separates the photoreceptors from their blood supply — builds up exponentially with age.21,22 The long-chain fatty acid, docosahexaenoic acid (DHA) is in high concentrations in the photoreceptor outer segments and is constantly shed and turned over during the normal visual cycle. A deficiency of DHA might therefore impair retinal function and promote AMD.23 Thus, there is a particular interest in research into fatty acids and AMD.
Results from two observational studies from the United States have led to recent controversial conclusions and recommendations regarding dietary fats. The first study, published in 2001, was a retrospective case–control study of 339 cases and 504 controls, suggesting that there was a higher risk of late AMD with higher vegetable fat intake (odds ratio [OR], 2.22; 95% CI, 1.32–3.74), and a lower risk with higher fish intake.24 In 2003, the same team looked at progression of disease in a prospective cohort study of 261 patients with early AMD. After an average follow-up of 4.6 years, they found a significant association between total fat intake (relative risk [RR], 2.74; 95% CI, 1.10–6.62) and vegetable fat intake (RR, 2.43; 95% CI, 1.10–5.37) and a higher risk of AMD progression.25 Higher saturated fat intake was also positively associated with AMD progression (RR, 1.96; 95% CI, 0.85–4.53), although this association was not statistically significant.
However, another US study, a 10-year retrospective population-based cohort study of 2429 participants, found an increased prevalence of AMD in those with the highest intake of saturated fats (mainly animal fats) (OR, 1.8; 95% CI, 1.2–2.7, P trend = 0.01). They also found the strongest association of increased risk of AMD in those with the highest butter intake (OR 1.5; 95% CI, 1.1–2.1, P trend = 0.006), although vegetable fats as a group were not investigated.26
Another 12-year pooled prospective cohort study of 72 489 participants revealed an increased risk of AMD in those with the highest intake of total fat (RR, 1.54; 95% CI, 1.17–2.01, P trend = 0.008); and a reduced risk of AMD in those with more servings of fish per week (RR, 0.65; 95% CI, 0.46–0.91, P trend = 0.009). However, the investigators did not find a statistically significant result for either animal fat (RR, 1.19; 95% CI, 0.87–1.62) or vegetable fat (RR, 1.33; 95% CI, 0.95–1.87) and the risk of AMD.27
The US studies on dietary fats need to be interpreted cautiously before applying them to an Australian diet. The increased risk associated with vegetable fats, reported in the articles by Seddon et al,24,25 is postulated to be due to the high levels of trans fatty acids (TFAs) in US margarines.
TFAs are formed industrially when liquid unsaturated fatty acids are hardened through a process of partial hydrogenation. These partially hydrogenated fats remain solid at room temperature and are more resistant to oxidation and spoilage, so they have been commonly used in shortenings, some margarines, and industrial cooking oils. In the 1990s, in Australia, concern about TFAs’ adverse effects on blood lipids led manufacturers to greatly reduce levels of TFAs in margarines. US margarines, on the other hand, still contain far greater quantities of TFAs.28 Therefore, the results of US studies on dietary fat intake may not be applicable to other countries like Australia.
In the Australian Blue Mountains Eye Study, a cross-sectional population-based study, a borderline statistically significant increased risk of early AMD with higher total fat intake (OR, 1.6; P trend = 0.08) and monounsaturated fat intake (OR, 1.5; P trend = 0.05) was found. There was also an increased risk (OR, 2.7; P trend = 0.04) of late AMD with higher cholesterol intake (animal fat).29 Although vegetable fats as a group were not investigated, they found polyunsaturated fats, which are derived mainly from vegetable fats, to be protective (OR, 0.4; P trend = 0.07) for late AMD. More recently, results from another Australian prospective cohort study found no relationship between polyunsaturated fats (vegetable fats) and incident cases of AMD, but found omega-3 fatty acids to be protective for early AMD (OR, 0.4; 95% CI, 0.2–0.8).30
The antioxidant carotenoids, lutein and zeaxanthin, found in dark green or yellow vegetables, exist naturally in high concentrations in the macula and have been proposed to play a protective role against the development of AMD.31 While the exact protective role of these macular pigments remains uncertain, it has been hypothesised that they may limit the damaging photo-oxidative effects of blue light through its absorption, and, because of their antioxidant effects, protect against the adverse effects of photochemical reactions.32 Aside from lutein and zeaxanthin, other antioxidants of interest include vitamins C and E, β-carotene, and the trace element zinc. PubMed and MEDLINE searches from 1966 until October 2005 revealed 23 observational studies and nine controlled trials evaluating dietary antioxidant intake (either individually or in combination) and the risk of AMD, with inconsistent results.
Of the nine controlled trials, three suggested that supplementation with various combinations of carotenoids and other antioxidants was protective. The largest and best conducted RCT from the Age Related Eye Disease Study (AREDS) group investigated the use of antioxidants (high doses of vitamin C, 500 mg; vitamin E, 400 IU; β-carotene, 15 mg) and zinc, 80 mg, with progression of AMD.33 They followed up 3640 participants for an average of 6.3 years. They showed that the use of antioxidants and/or zinc in the 2577 participants with a high-risk of AMD (AREDS category 3 or 4) resulted in a reduced risk of disease progression of between 17% to 25% when compared with placebo, with the greatest risk reduction in those taking the combination of antioxidants and zinc. No benefit was seen in lower-risk AMD groups (AREDS category 1 or 2).
There has been much debate about the interpretation of the AREDS data and the appropriateness of the widespread use of this high-dose supplement. However, these data have been widely distributed to people at risk of visual loss from AMD. As increased risk of lung cancer among smokers taking β-carotene supplements34,35 was noted during the study, the AREDS formulation is not recommended for smokers.
In Australia, the Macu-Vision supplement (Blackmores, Sydney, NSW), produced to mimic the AREDS formulation, excluded β-carotene due to the health risks. Though only one tablet is recommended on the bottle, two tablets need to be taken to achieve the same level of supplementation used in AREDS. In Australia, a recent study of 100 patients with high-risk AMD revealed that 38% were taking supplements, but only 1% of them were taking the correct dose.36
Among the other six trials which did not show any associations between zinc, vitamin E and other multivitamin intake with AMD, we will highlight two of the larger and better conducted trials: (1) VECAT (vitamin E, cataract, and age-related maculopathy trial), an RCT of 1193 participants randomised to 500 IU of vitamin E or placebo, revealed no benefit (RR, 1.05; 95% CI, 0.69–1.61) for early AMD.37 (2) the ATBC (α-tocopherol and β-carotene) study, an RCT of 29 000 males randomly assigned to receive vitamin E 50 mg/day; β-carotene 20 mg/day; both vitamin E 50 mg/day; and β-carotene 20 mg/day; or placebo, from which a randomly selected sample of 941 participants from the original cohort underwent an ophthalmological examination, did not reveal beneficial effects from the 6 years of supplementation.38
- Robyn H Guymer1
- Elaine Wei-Tinn Chong2
- Department of Ophthalmology, Centre for Eye Research Australia, University of Melbourne, Melbourne, VIC.
None identified.
- 1. McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol 2001; 119: 1455-1462.
- 2. Access Economics. Clear insight. The economic impact and cost of visual loss in Australia. Melbourne: Eye Research Australia, 2004. Available at: http://iris.medoph.unimelb.edu.au/new/clearinsight/pdf/ae1_final.pdf (accessed Mar 2006).
- 3. Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 2002; 109: 1767-1779.
- 4. Vingerling JR, Hofman A, Grobbee DE, et al. Age-related macular degeneration and smoking. The Rotterdam Study. Arch Ophthalmol 1996; 114: 1193-1196.
- 5. Thornton J, Edwards R, Mitchell P, et al. Smoking and age-related macular degeneration: a review of association. Eye 2005; 19: 935-944.
- 6. Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol 1986; 104: 216-219.
- 7. Goldberg J, Flowerdew G, Smith E, et al. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol 1988; 128: 700-710.
- 8. Vidaurri JS, Pe’er J, Halfon ST, et al. Association between drusen and some of the risk factors for coronary artery disease. Ophthalmologica 1984; 188: 243-247.
- 9. Hyman L, Schachat AP, He Q, et al. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000; 118: 351-358.
- 10. Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1993; 100: 406-414.
- 11. Smith W, Mitchell P, Leeder SR, et al. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol 1998; 116: 583-587.
- 12. Schaumberg DA, Christen WG, Hankinson SE, et al. Body mass index and the incidence of visually significant age-related maculopathy in men. Arch Ophthalmol 2001; 119: 1259-1265.
- 13. Klein BE, Klein R, Lee KE, et al. Measures of obesity and age-related eye diseases. Ophthalmic Epidemiol 2001; 8: 251-262.
- 14. Chaine G, Hullo A, Sahel J, et al. Case-control study of the risk factors for age related macular degeneration. France-DMLA Study Group. Br J Ophthalmol 1998; 82: 996-1002.
- 15. Vingerling JR, Dielemans I, Bots ML, et al. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol 1995; 142: 404-409.
- 16. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol 1997; 115: 741-747.
- 17. Guymer RH, Chiu AW, Lim L, et al. HMG CoA reductase inhibitors (statins): do they have a role in age-related macular degeneration? Surv Ophthalmol 2005; 50: 194-206.
- 18. Klein R, Klein BE, Tomany SC, et al. Relation of statin use to the 5-year incidence and progression of age-related maculopathy. Arch Ophthalmol 2003; 121: 1151-1155.
- 19. McCarty CA, Mukesh BN, Guymer RH, et al. Cholesterol-lowering medications reduce the risk of age-related maculopathy progression [letter]. Med J Aust 2001; 175: 340.
- 20. Guymer RH, Robman L, Varsamidis M, et al. Can HMG Co-A reductase inhibitors (“Statins”) slow the progression of AMD? In: Association for Research in Vision and Ophthalmology Abstract No. 2364; 2005. Fort Lauderdale, Florida: ARVO, 2005.
- 21. Sheraidah G, Steinmetz R, Maguire J, et al. Correlation between lipids extracted from Bruch’s membrane and age. Ophthalmology 1993; 100: 47-51.
- 22. Ruberti JW, Curcio CA, Millican CL, et al. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch’s membrane. Invest Ophthalmol Vis Sci 2003; 44: 1753-1759.
- 23. Vinding T, Appleyard M, Nyboe J, et al. Risk factor analysis for atrophic and exudative age-related macular degeneration. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 1992; 70: 66-72.
- 24. Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 2001; 119: 1191-1199.
- 25. Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003; 121: 1728-1737.
- 26. Mares-Perlman JA, Brady WE, Klein R, et al. Dietary fat and age-related maculopathy. Arch Ophthalmol 1995; 113: 743-748.
- 27. Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr 2001; 73: 209-218.
- 28. Mansour MP, Sinclair AJ. The trans fatty acid and positional (sn-2) fatty acid composition of some Australian margarines, dairy blends and animal fats. Asia Pac J Clin Nutr 1993; 2: 155-163.
- 29. Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol 2000; 118: 401-404.
- 30. Chua B, Flood V, Rochtchina E, et al. Dietary fatty acids and the 5-year incidence of age related maculopathy. Arch Ophthalmol 2006. In press.
- 31. Mozaffarieh M, Sacu S, Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr J 2003; 2: 20.
- 32. Alves-Rodrigues A, Shao A. The science behind lutein. Toxicol Lett 2004; 150: 57-83.
- 33. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001; 119: 1417-1436.
- 34. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996; 334: 1150-1155.
- 35. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994; 330: 1029-1035.
- 36. Ng WT, Goggin M. Awareness of and compliance with recommended dietary supplement among age-related macular degeneration patients. Clin Experiment Ophthalmol 2006; 34: 9-14.
- 37. Taylor HR, Tikellis G, Robman LD, et al. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ 2002; 325: 11.
- 38. Teikari JM, Laatikainen L, Virtamo J, et al. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand 1998; 76: 224-229.





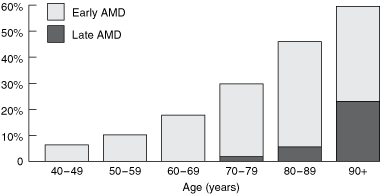
Abstract
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in Australia and other Western countries.
As there is no cure for AMD, and treatments to stop its progression have met with limited success, there is an interest in identifying modifiable risk factors to prevent or slow disease progression.
To date, smoking is the only proven modifiable risk factor for AMD. Other factors under study include (i) cardiovascular risk factors such as hypertension, body mass index, and atherosclerosis; and (ii) dietary risk factors including fat and antioxidant intake, but so far these studies have produced conflicting results.
Dietary fat in relation to AMD has recently attracted media attention. Despite very limited work supporting an association between vegetable fat and AMD, widespread publicity advocating margarine as a cause of AMD and encouraging use of butter instead has caused confusion and anxiety among sufferers of AMD and the general public, as well as concern among health professionals.
The antioxidant carotenoids — lutein and zeaxanthin — found in dark green or yellow vegetables exist in high concentrations in the macula and are hypothesised to play a protective role. Of nine controlled trials of supplementation with carotenoids and other antioxidants, three suggested that various combinations of antioxidants and carotenoids were protective.
While a low-fat diet rich in dark green and yellow vegetables is advocated in general, any specific recommendations regarding certain fats or antioxidant supplementation and AMD are not based on consistent findings at this stage.