There is strong evidence, most notably from the United Kingdom Prospective Diabetes Study (UKPDS),1,2 that the main goal in treating type 2 diabetes should be to achieve blood glucose levels as close as possible to the non-diabetic range to prevent chronic microvascular and macrovascular complications. This evidence is recognised by key national bodies, such as the Australian Diabetes Society3 and the American Diabetes Association,4 which recommend a target glycated haemoglobin (HbA1c) level of < 7.0%. As epidemiological analyses reveal no glycaemic threshold for vascular benefit, more stringent goals (including HbA1c < 6.0%) might be considered if the clinical situation allows.4 The progressive nature of type 2 diabetes means that patients require periodic intensification of blood glucose-lowering therapy with these goals in mind. For example, projections from UKPDS data indicate that most patients will need insulin therapy to maintain a target HbA1c level of < 7.0% after 9 years of diagnosed type 2 diabetes.5
Although ample data describe the temporal relationship between glycaemia and intensification of therapy in protocol-driven intervention studies such as the UKPDS, little is known of real-life management in the community. This is especially true of insulin therapy, which patients and prescribing physicians can be reluctant to consider, even when maximal doses of oral hypoglycaemic agents (OHA) have become ineffective.6,7
We analysed data from the FDS,8,9 a longitudinal observational study of a representative sample of patients from an area (population, 120 097) surrounding the port of Fremantle in Western Australia. Recruitment strategies, sample characteristics and details of identified but non-recruited patients have been described previously.8 The FDS recruited 1294 patients with type 2 diabetes between 1993 and 1996. Annual follow-up assessments continued until 2001. The current analysis included the 531 patients (41% of those recruited) who attended the baseline assessment and five subsequent consecutive annual FDS review visits.
At each FDS visit, demographic and clinical information, including details of diabetes management, was documented. Participants underwent a standard clinical examination, and biochemical tests were done on fasting blood and first-morning urine samples.8
The computer package SPSS for Windows (version 11.5; SPSS Inc, Chicago, Ill, USA) was used for data analysis. Data are presented as proportions, means (95% CI) or, for variables which did not conform to a normal or log-normal distribution, medians (interquartile range [IQR]). Comparison of paired data was by paired t test, McNemar’s test, and the Wilcoxon signed-rank test. The Kruskal–Wallis test was used to compare several independent samples.10
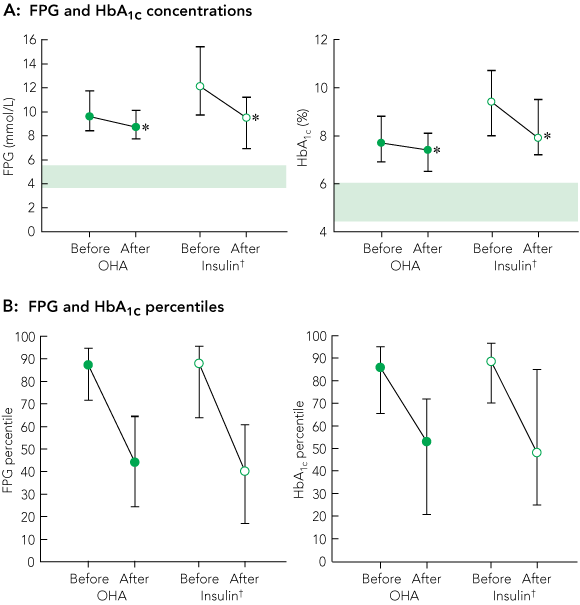
Median FPG and HbA1c concentrations at the annual FDS visits immediately before and after intensification of therapy are shown in Box 1A, and concomitant changes in BMI, blood pressure and serum lipid levels are summarised in Box 2.
FPG and HbA1c concentrations of each patient were ranked within their treatment group for each FDS visit and converted to percentiles. For patients changing therapy, FPG and HbA1c percentiles were pooled for each treatment group for the visits before and after the change (Box 1B). The median percentile rank was over 86% for both measures and all treatment groups before therapy change, and dropped to between 40% and 53% after therapy change.
It could be argued that glycaemic targets for type 2 diabetes were less ambitious during the first 5 years of our study than later, as the key results of the UKPDS were not released until 1998.1 However, we found no effect of calendar year on thresholds for therapeutic change in our cohort. Furthermore, the Australian Diabetes Society recommended strict metabolic control (HbA1c < 7.0%) for patients with type 2 diabetes in 1993, after publication of the results of the Diabetes Control and Complications Trial in type 1 diabetes.3 Indeed, the US National Health and Nutrition Epidemiologic Surveys found a decrease between 1988 and 2000 in the proportion of patients with diabetes achieving an HbA1c level < 7.0%, despite the recommendation for more intensive treatment regimens.11
As might be expected from the improved glycaemic control in our patients after therapy was intensified, fasting serum triglyceride concentrations fell significantly.12 We did not observe any change in blood pressure, apart from a mean 3 mmHg reduction in diastolic blood pressure in patients starting insulin, which appeared independent of the effect of antihypertensive therapy.
Insulin initiation has been shown to have variable effects on blood pressure,13 including benefits, but may also lead to increased blood pressure which accompanies weight gain.14 Our participants had not gained significant weight by the time of the next FDS visit after intensification of therapy (an average of 6 months later), even after the introduction of insulin. This might indicate a greater readiness to revisit lifestyle modification in our community-based rather than clinic-based participants, but it could also reflect the fact that the levels of glycaemia in our participants did not fall to those associated with a marked reduction in glycosuria. Nevertheless, we found that mean BMI among participants who progressed from OHA to insulin increased significantly after a further year of insulin treatment. This finding is consistent with UKPDS data showing a 2–4 kg body weight increase in the first 1–2 years after randomisation to insulin in intensively-treated patients.1
Our data provide some evidence of a link between reduced compliance and therapeutic progression in diabetes, as found in a US study.13 In our study, the percentage of OHA-treated patients who were fully compliant was greater among those who had just begun OHA (an average of 6 months earlier) than among those who were subsequently started on insulin therapy (the latter having 3–4 years longer duration of diabetes). Those who progressed to insulin therapy had better compliance (over 90%) when it was introduced. However, it is likely that the trend to reduced compliance over time might also occur in this group. Indeed, in a large US study involving a Department of Veterans Affairs regional database of patients using long-term insulin therapy, only 77% adhered to prescribed regimens.16
Our data are consistent with the results of shorter-term studies in other contexts. In a primary-care cohort of patients with type 2 diabetes in the UK, identification of HbA1c above the target level of 8.0% led to intensification of therapy in about 50% of cases.17 In other studies of managed-care patients in the US,18,19 initiation of new therapies produced a fall in HbA1c, but fewer than 20% of patients had a level below 7.0% during the subsequent 1–2 years. In the UK primary-care study, there appeared to be a particular reluctance to prescribe insulin.17
The results of studies from other countries11,17-19 and our current data provide strong evidence that most patients with type 2 diabetes spend most of the duration of their disease with suboptimal glycaemic control based on the currently recommended HbA1c target of < 7.0%. This is despite the availability of effective therapies, which could be introduced earlier and which, on the basis of the present study, may not increase the risk of hypoglycaemia. Our results indicate that the clear messages of studies such as the UKPDS are not being heeded in Australia.
1 Fasting plasma glucose (FPG) and glycated haemoglobin (HbA1c) before and after introduction of oral hypoglycaemic agents (OHA) or insulin
Received 18 August 2005, accepted 9 February 2006
- Timothy M E Davis
- Wendy A Davis
- David G Bruce
- School of Medicine and Pharmacology, University of Western Australia, Fremantle, WA.
The Fremantle Diabetes Study (FDS) was funded by the Raine Foundation, University of Western Australia, Perth, WA. We thank the FDS research staff for their assistance with data collection, and the Biochemistry Department, Fremantle Hospital, Fremantle, WA, for performing biochemical tests.
None identified.
- 1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853.
- 2. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405-412.
- 3. Yue DK, Colagiuri S, McElduff A, Silink M. Diabetes Control and Complications Trial. Position statement of the Australian Diabetes Society. Med J Aust 1993; 159: 803-804.
- 4. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28 Suppl 1: S4-S36.
- 5. Turner RC, Cull CA, Frighi V, Holman RR for the UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281: 2005-2012.
- 6. Polonsky WH, Jackson RA. What’s so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes 2004; 22: 147-150. Available at: http://clinical.diabetesjournals.org/cgi/reprint/22/3/147 (accessed Feb 2006).
- 7. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 2002; 26 Suppl 3: S18-S24.
- 8. Davis TME, Zimmett P, Davis WA, et al. Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multiethnic Australian community: the Fremantle Diabetes Study. Diabetic Med 2000; 17: 667-674.
- 9. Bruce DG, Davis WA, Davis TME. Glycemic control in elderly subjects with type 2 diabetes mellitus in the Fremantle Diabetes Study. J Am Geriatr Soc 2000; 48: 1449-1453.
- 10. Dawson-Saunders B, Trapp RG. Basic and clinical biostatistics. Connecticut, USA: Appleton and Lange, 1990.
- 11. Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27: 17-20.
- 12. Steinmetz A. Treatment of diabetic dyslipoproteinemia. Exp Clin Endocrinol Diabetes 2003; 111: 239-245.
- 13. Riddle MC. Different takes on the relationship of insulin treatment to blood pressure. Diabetes Care 1993; 16: 953-954.
- 14. Genev NM, Lau IT, Willey KA, et al. Does insulin therapy have a hypertensive effect in type 2 diabetes? J Cardiovasc Pharmacol 1998; 32: 39-41.
- 15. Kogut SJ, Andrade SE, Willey C, Larrat EP. Nonadherence as a predictor of antidiabetic drug therapy intensification (augmentation). Pharmacoepidemiol Drug Saf 2004; 13: 591-598.
- 16. Cramer JA, Pugh MJ. The influence of insulin use on glycemic control: how well do adults follow prescriptions for insulin? Diabetes Care 2005; 28: 78-83.
- 17. Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabet Med 2004; 21: 150-155.
- 18. Karter AJ, Moffet HH, Liu J, et al. Achieving good glycemic control: initiation of new antihyperglycemic therapies in patients with type 2 diabetes from the Kaiser Permanente Northern California Diabetes Registry. Am J Manag Care 2005; 11: 262-270.
- 19. Hayward RA, Manning WG, Kaplan SH, et al. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA 1997; 278: 1663-1669.





Abstract
Objective: To assess the effectiveness of the management of type 2 diabetes in an urban Australian setting.
Design and setting: The Fremantle Diabetes Study (FDS), a community-based longitudinal observational study.
Patients: 531 FDS participants with type 2 diabetes, with mean age, 62.4 years (95% CI, 40.9–79.3 years), 54% male, median diabetes duration 3.0 years (interquartile range [IQR], 0.7–7.0 years), with valid data from the baseline FDS assessment and five subsequent annual reviews between 1993 and 2001.
Main outcome measures: Glycated haemoglobin (HbA1c) levels at annual review visits before and after change in blood glucose-lowering therapy.
Results: Over 2893 patient-years of follow-up, 97 patients (18%) progressed from dietary management to therapy with oral hypoglycaemic agents (OHA), and 45 (9%) progressed from OHA to insulin therapy, after a median duration of diabetes of 4.0 years (IQR, 2.9–5.5 years) and 8.1 years (IQR, 5.5–13.0 years), respectively. Median HbA1c concentrations (IQR) at the review before OHA or insulin were started were 7.7% (6.9%–8.8%) and 9.4% (8.0%–10.7%), respectively. At the next annual review, HbA1c levels in the two groups had fallen to 7.4% (6.5%–8.1%) and 7.9% (7.2%–9.5%), respectively (P ≤ 0.001). Intensification of therapy was associated with beneficial changes in serum lipid profiles, but not with an increase in frequency of hypoglycaemia.
Conclusions: Most Australian patients with type 2 diabetes may be spending most of the duration of their disease with suboptimal glycaemic control (HbA1c > 7.0%), despite the availability of a range of effective therapies, including insulin.