Rheumatoid arthritis (RA) affects 1% of the population, two-thirds of whom will develop erosive joint disease, and has a peak incidence at 20–40 years of age. After 10 years of disease, 50% of people with RA are unable to work because of disability. Mortality rates are also increased, with RA patients’ average life expectancy being reduced by 10 years. The major cause of this premature death appears to be accelerated atherosclerosis.1
Over the past 10 years, the traditional nihilistic view of RA treatment has been replaced by an evidence-based “attitude” of therapeutic opportunity.2 This change has been achieved through the use of the class of medications known as disease modifying antirheumatic drugs (DMARDs; Box 1) and is the result of three major advances: early use of DMARDs, use of DMARDs in combination, and the advent of new DMARDs, including biological DMARDs. Most people who develop RA today can expect to have their disease well controlled and are likely to avoid severe long-term damage and disability. In most cases, the need to continue medication is lifelong, as RA will generally relapse on medication withdrawal.21
Despite the evidence of DMARD effectiveness, barriers to implementation remain. The way forward may require broader appreciation (among both primary care physicians and the public) of the benefits of the revolution in treatment in RA and logistic changes in the way specialist rheumatologists and arthritis clinics triage appointments. These barriers to implementation have been highlighted by the Australian National Health Priority for Arthritis and Musculoskeletal Conditions as a priority issue (NHPAC Action Plan for Arthritis).
Levels of evidence in the following review are derived from the National Health and Medical Research Council’s system for assessing evidence.22
Methotrexate has become the first-line treatment for all but the mildest RA, and is the standard against which other treatments are compared. As an example of its ascendancy, in one academic rheumatology setting in 1985 only 10% of RA patients were taking methotrexate, compared with 76% in 2000.23 As monotherapy, methotrexate reduces disease activity and the rate of joint erosions (E1).3 In addition, methotrexate has been associated with a substantial reduction in long-term mortality in RA patients. This survival benefit was not provided by sulfasalazine, hydroxychloroquine, gold or penicillamine (E3-2).24 The safety profile of low dose weekly methotrexate is entirely acceptable compared with other options (Box 1), based on experience with its widespread use since the mid 1980s. Concerns regarding progressive liver damage as a consequence of weekly low dose methotrexate have not been realised, and most patients continue on methotrexate for many years, providing further evidence of its favourable risk–benefit profile.25
Although there have been proponents of early DMARD use for many years, withholding DMARDs until radiographic joint erosions had occurred was considered reasonable as recently as 12 years ago.26 The current widespread acceptance of an early DMARD approach2 has been driven by complementary streams of evidence. First, joint damage occurs early in the disease process. Seventy-five per cent of joint erosions occur within the first 2 years, and at least 25% of patients already have erosions at disease diagnosis.27 Second, early DMARD use decreases rates of joint damage compared with delayed DMARD use.2 Delays of even a few months in commencing DMARD treatment may result in a more aggressive disease course in the first year.28 Third, early DMARD use appears to make the disease easier to control in the long term (Box 2).29 Last, DMARD treatment is remarkably safe when used correctly.25
Current recommendations suggest commencing DMARD treatment at the time of RA diagnosis.2 Although diagnostic certainty is more difficult to achieve in early RA than in more established disease, the safety of treatment justifies early DMARD treatment, and early diagnosis is usually achievable in specialist early arthritis clinics or community-based rheumatology settings. As more than 90% of patients with recent onset polyarthritis of more than 12 weeks’ duration can be expected to progress to established RA,30 almost everyone treated with DMARDs at that stage can be expected to benefit.
Of more practical concern is the sometimes difficult distinction between early RA and non-threatening chronic rheumatic conditions. The synovitis that is usually present in early RA is a critical discriminator, but non-specialists may not reliably detect it,31 highlighting the need for referral if there is any doubt. Fortunately, recent developments may increase diagnostic confidence in early disease. New diagnostic guidelines for early RA that determine the likelihood of persistence and erosiveness are being validated.32 In specialist clinical settings, magnetic resonance imaging and ultrasonography can confirm the presence of joint synovitis when there is clinical uncertainty, and detect joint erosions well before they are visible on radiographs.33 Antibodies against cyclic citrullinated peptide appear to be specific for RA, with a high positive predictive value.34 In future, novel biomarkers are likely to provide additional diagnostic and prognostic certainty, and may allow targeting of specific DMARD treatments to individuals.32
Different combinations of DMARD treatments have been studied in randomised controlled trials (Box 1).2 Generally, these trials have included patients with ongoing disease activity despite adequate methotrexate treatment. The message from these trials is that patients who do not respond to DMARD monotherapy are likely to respond to the addition of a second and sometimes a third DMARD, or so-called step-up therapy. This is a change from previous practice, in which DMARDs were substituted sequentially (ie, serial monotherapy). Furthermore, there is evidence that combination DMARD therapy from the outset is superior to DMARD monotherapy in inducing remission and preventing joint damage.35 Although the specific details of the best DMARD protocol remain to be elucidated and must often be tailored to the individual, early combination DMARD therapy in active RA is the most effective strategy for disease suppression and containment of joint damage (E2).
Perhaps surprisingly, the tolerability and toxicity of combination treatment was no different from that of monotherapy in these short-term trials. One caveat is that randomised controlled trials are not able to accurately measure the rate of “serious but rare” side effects because of their small sample size. In addition, the long-term risks of combination DMARD therapy in RA are not known. On the other hand, the long-term risks of uncontrolled RA are substantial. The balance of these benefits and risks has lead to recommendations to implement combination DMARD treatment in early active RA.2
In the past decade, cyclosporin, leflunomide, and the biological DMARDs, including the tumour necrosis factor (TNF) inhibitors infliximab, etanercept, and adalimumab, and the interleukin 1 receptor inhibitor anakinra, have become available in Australia for the treatment of RA. These agents differ in effectiveness and risk (Box 1). Used as monotherapy, and more especially when used in combination with methotrexate, the TNF inhibitors have added substantially to the range of effective agents for RA.
Individual tolerance and response to all available agents varies. Treatment plans need to be guided by (i) dosage adjustments according to objective response criteria, (ii) disease remission as the treatment goal, (iii) monitoring for known and emergent intolerances, and (iv) cost. For example, the annual prescription cost of methotrexate is of a similar order to the average daily cost for a bDMARD.
The evidence outlined above provides solid support for early diagnosis and combination DMARD therapy in RA. Meeting the challenge of this evidence requires improvements in early recognition in primary care, improvements in systems to provide timely evaluation of people with possible early RA, and orderly approaches to management.
There are several reasons for difficulty in making an early diagnosis. First, inflammatory arthritis is a common manifestation of many conditions, some of which are generally benign and/or self-limiting (eg, viral arthritis, osteoarthritis), while others are more serious (eg, systemic vasculitis, infective endocarditis). However, investigations such as viral screens at presentation, and special investigations triggered by clinical suspicion are often helpful to exclude other diagnoses. Second, the “classical” clinical pattern of RA with persistent symmetrical small and large joint arthropathy tends to emerge over time, with a migratory (“palindromic rheumatism”) or incomplete pattern often present in the first few months or even years. Third, diagnostic criteria or tests that may be useful in established disease, such as rheumatoid factor and hand radiographs, are more likely to be negative in early disease. As a diagnostic test for RA, rheumatoid factor is neither very sensitive nor specific. The assay for cyclic citrullinated peptide antibodies (anti-CCP) may be more informative.34 However, over-reliance on negative results from these tests may delay referral, diagnosis, and effective DMARD treatment. Fourth, symptoms and signs may be masked by treatment with anti-inflammatory drugs or corticosteroids.
The limited Australian data on diagnostic and treatment delays in early RA indicate problems in recognition and timely referral at the primary care level, as well as delays between referral and first specialist assessment. For example, at a large Melbourne public hospital outpatient department in 2002, the median delay between symptom onset and referral was more than 10 months, and the median delay between referral and first specialist assessment was almost 2 months.36 In contrast, a survey of community-based rheumatologists in the same geographic area showed a delay of 3 months between symptom onset and referral, and 1 month between referral and first specialist assessment.
Timeliness of access to specialist services will vary depending on the location, and large variability is likely to exist between urban and rural settings. Thus, a more systematic approach to triage and referral may ensure that most patients with RA have rapid access to best-practice treatment.
Timeliness of access to specialty assessment may deteriorate further as consultant rheumatologists struggle to keep pace with the burgeoning clinical demands of ageing and chronic arthritic disease. This potential crisis may mandate triage systems which focus specialist resources on clinical priorities, such as RA, where treatment is complex, and the gap between outcomes from best practice and non-expert interventions is greatest. This philosophy, which has its antecedents in coronary care and emergency units, has encouraged the establishment of Early Arthritis Clinics. These clinics have successfully reduced delays in instituting DMARD treatment in other countries by applying a structured triage process, prompt specialist clinical assessment and diagnosis, pre-determined default evidence-based treatment plans, and regular communication links with primary care clinicians.31 With appropriate organisation, this manner of coordinated and structured health care can be delivered by community-based rheumatologists. These community-based services, which form the backbone of rheumatology care, may be organised on a “standalone” basis or through collaborations with pioneering teaching hospital-based services. The more orderly management approaches inherent in such services provide opportunities to delegate tasks to practice nurses and assistants, thereby achieving economies without compromising care.37
Successful triage systems require an accurate clinical assessment in the primary care setting. A simple system has been successfully trialled in a community setting in England and is likely to be useful in Australia (Box 3).38 In general, the following findings are suggestive of early rheumatoid arthritis and warrant specialist referral: recent onset of joint swelling, symmetrical symptoms, metacarpophalangeal or metatarsophalangeal involvement, significant early morning stiffness, a good response to non-steroidal anti-inflammatory drugs, and a family history of rheumatoid arthritis. Initial treatment with a non-steroidal anti-inflammatory agent can provide symptomatic relief, which is of some diagnostic utility. Glucocorticoid use is not encouraged. Glucocorticoids are such potent anti-inflammatory and mood-altering agents that short-term relief is nonspecific, their capacity to suppress joint swelling and acute phase response adds to diagnostic difficulties, and their use may deflect from or delay the application of more effective DMARD therapy.
There have been important advances in the treatment of RA in the past 10 years. Early treatment with combinations of DMARDs provides the most effective disease control and prevention of long-term joint damage. This strategy is also likely to result in substantial downstream health cost savings as disability and workforce-withdrawal are reduced. Newer DMARDs have increased the therapeutic armamentarium such that for most people with RA, a safe and effective treatment is available. In addition to early arthritis clinics, improved triage systems are required to help the health system meet the challenge of universal early diagnosis and treatment of RA.
1 Efficacy, safety and monitoring principles for disease modifying antirheumatic drugs (DMARDs) in the treatment of rheumatoid arthritis
DMARD |
Efficacy |
Efficacy in combination with methotrexate |
Major toxicity frequency3 |
Usual routine monitoring for toxicity3 |
|||||||||||
Methotrexate |
++4 |
— |
+ |
FBC, LFT |
|||||||||||
Sulfasalazine |
++5 |
+++6,7 |
+ |
FBC, LFT |
|||||||||||
Hydroxychloroquine |
+8 |
++6 |
- |
Retinal examination |
|||||||||||
Leflunomide |
++9 |
+++10 |
+ |
FBC, LFT |
|||||||||||
Tumour necrosis factor inhibitors |
++11 |
+++11-13 |
+ |
FBC, E+C, LFT |
|||||||||||
Anakinra |
+ |
++14 |
+ |
FBC, E+C, LFT |
|||||||||||
Intramuscular gold |
++15 |
+++16 |
++ |
FBC, Urinalysis |
|||||||||||
Cyclosporin |
++17 |
+++18 |
++ |
FBC, E+C, LFT, BP |
|||||||||||
Azathioprine |
+19 |
Unknown |
+ |
FBC, LFT |
|||||||||||
Cyclophosphamide |
++19 |
Not used |
+++ |
FBC, E+C, LFT, urinalysis |
|||||||||||
d-Penicillamine |
++20 |
Unknown |
+++ |
FBC, E+C, LFT, urinalysis |
|||||||||||
+/– scoring is a clinical guide to relative effects representing the views of the authors based on published studies where indicated. +++ represents the maximum efficacy or toxicity. FBC = full blood count. LFT = liver function tests. E+C = electrolytes and creatinine. BP = blood pressure. |
|||||||||||||||
2 Potential average effect of early or late disease modifying antirheumatic drug treatment on the activity of rheumatoid arthritis over time
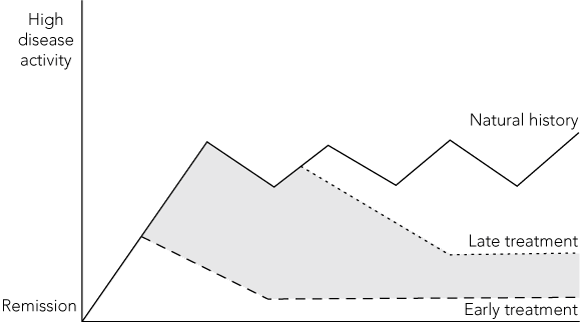
Early treatment appears to improve remission rates.29 Shaded area under the curve represents permanent joint damage that accrues during periods of uncontrolled disease activity.
3 Triage system for identification of possible early rheumatoid arthritis 38
Any positive findings in the following three features might warrant referral for early arthritis assessment:
three or more swollen joints
early morning joint stiffness > 30 minutes
bilateral metatarsophalangeal or metacarpophalangeal squeeze tenderness
AND arthritis (pain) duration < 1 year
- Lynden J Roberts1
- Leslie G Cleland2
- Susanna M Proudman3
- Ranjeny Thomas4
- 1 Departments of Medicine and Rheumatology, University of Melbourne, Parkville, VIC.
- 2 Rheumatology Unit, Royal Adelaide Hospital, Adelaide, SA.
- 3 Centre for Immunology and Cancer Research, University of Queensland, Woolloongabba, QLD.
We thank Professor Ian Wicks, Associate Professor Geoffrey McColl, Drs Sharon Van Doornum, Caroline Brand, Phillip Vecchio, Lisa Carroll, Mukhlesur Rahman, Paul Kubler, Catherine L Hill, Anita Lee, Fiona Goldblatt, Professor Michael James and members of the Optimal Management of Early Rheumatoid Arthritis Network (OMERAN) for their contribution to the development of concepts, protocols and criteria for early RA clinics in Australia. The authors were supported by grants from the University of Melbourne (L R), NHMRC (L C, S P, R T), RAH Special Purposes Fund (L C, S P), Princess Alexandra Hospital Foundation, Rotary Centenary Project, and Arthritis Queensland (R T).
None identified.
- 1. Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum 2002; 46: 862-873.
- 2. O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med 2004; 350: 2591-2602.
- 3. GUIPCAR Group. Clinical practice guideline for the management of rheumatoid arthritis in Spain. Madrid: Spanish Society of Rheumatology, 2001.
- 4. Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312: 818-822.
- 5. Choy EH, Scott DL, Kingsley GH, et al. Treating rheumatoid arthritis early with disease modifying drugs reduces joint damage: a randomised double blind trial of sulphasalazine vs diclofenac sodium. Clin Exp Rheumatol 2002; 20: 351-358.
- 6. O’Dell JR, Haire CE, Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 1996; 334: 1287-1291.
- 7. Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997; 350: 309-318.
- 8. HERA Study Group. A randomized trial of hydroxychloroquine in early rheumatoid arthritis: the HERA Study. Am J Med 1995; 98: 156-168.
- 9. Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet 1999; 353: 259-266.
- 10. Kremer JM, Genovese MC, Cannon GW, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2002; 137: 726-733.
- 11. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004; 363: 675-681.
- 12. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999; 354: 1932-1939.
- 13. Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003; 48: 35-45.
- 14. Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis 2004; 63: 1062-1068.
- 15. Rau R, Herborn G, Menninger H, Sangha O. Progression in early erosive rheumatoid arthritis: 12 month results from a randomized controlled trial comparing methotrexate and gold sodium thiomalate. Br J Rheumatol 1998; 37: 1220-1226.
- 16. Lehman AJ, Esdaile JM, Klinkhoff AV, et al. A 48-week, randomized, double-blind, double-observer, placebo-controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: results of the METGO study. Arthritis Rheum 2005; 52: 1360-1370.
- 17. Yocum DE, Klippel JH, Wilder RL, et al. Cyclosporin A in severe, treatment-refractory rheumatoid arthritis. A randomized study. Ann Intern Med 1988; 109: 863-869.
- 18. Marchesoni A, Battafarano N, Arreghini M, et al. Radiographic progression in early rheumatoid arthritis: a 12-month randomized controlled study comparing the combination of cyclosporin and methotrexate with methotrexate alone. Rheumatology (Oxford) 2003; 42: 1545-1549.
- 19. Gaffney K, Scott DG. Azathioprine and cyclophosphamide in the treatment of rheumatoid arthritis. Br J Rheumatol 1998; 37: 824-836.
- 20. Jessop JD, O’Sullivan MM, Lewis PA, et al. A long-term five-year randomized controlled trial of hydroxychloroquine, sodium aurothiomalate, auranofin and penicillamine in the treatment of patients with rheumatoid arthritis. Br J Rheumatol 1998; 37: 992-1002.
- 21. Gotzsche PC, Hansen M, Stoltenberg M, et al. Randomized, placebo controlled trial of withdrawal of slow-acting antirheumatic drugs and of observer bias in rheumatoid arthritis. Scand J Rheumatol 1996; 25: 194-199.
- 22. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999. Available at: http://www.nhmrc.gov.au/publications/synopses/cp30syn.htm (accessed Jan 2006).
- 23. Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum 2005; 52: 1009-1019.
- 24. Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 2002; 359: 1173-1177.
- 25. Wolfe F. Adverse drug reactions of DMARDs and DC-ARTs in rheumatoid arthritis. Clin Exp Rheumatol 1997; 15 Suppl 17: S75-S81.
- 26. Williams HJ. Rheumatoid arthritis: treatment. In: Schumacher HR Jr, editor. Primer on the rheumatic diseases. 10th ed. Atlanta: Arthritis Foundation, 1993.
- 27. van der Horst-Bruinsma IE, Speyer I, Visser H, et al. Diagnosis and course of early-onset arthritis: results of a special early arthritis clinic compared with routine patient care. Br J Rheumatol 1998; 37: 1084-1088.
- 28. Nell VP, Machold KP, Eberl G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004; 43: 906-914.
- 29. Mottonen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum 2002; 46: 894-898.
- 30. Green M, Marzo-Ortega H, McGonagle D, et al. Persistence of mild, early inflammatory arthritis: the importance of disease duration, rheumatoid factor, and the shared epitope. Arthritis Rheum 1999; 42: 2184-2188.
- 31. Quinn MA, Emery P. Are early arthritis clinics necessary? Best Pract Res Clin Rheumatol 2005; 19: 1-17.
- 32. Visser H. Early diagnosis of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2005; 19: 55-72.
- 33. Ostergaard M, Ejbjerg B, Szkudlarek M. Imaging in early rheumatoid arthritis: roles of magnetic resonance imaging, ultrasonography, conventional radiography and computed tomography. Best Pract Res Clin Rheumatol 2005; 19: 91-116.
- 34. Raza K, Breese M, Nightingale P, et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol 2005; 32: 231-238.
- 35. Korpela M, Laasonen L, Hannonen P, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum 2004; 50: 2072-2081.
- 36. Reed MD, Moran H, McColl G, et al. Time to institution of DMARD therapy in Victoria — an early rheumatoid arthritis pilot study. Australian Rheumatology Association Annual Scientific Meeting. Melbourne, 22–26 May 2005.
- 37. Tijhuis GJ, Zwinderman AH, Hazes JM, et al. Two-year follow-up of a randomized controlled trial of a clinical nurse specialist intervention, inpatient, and day patient team care in rheumatoid arthritis. J Adv Nurs 2003; 41: 34-43.
- 38. Emery P, Breedveld FC, Dougados M, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002; 61: 290-297.





Abstract
Most people presenting with rheumatoid arthritis today can expect to achieve disease suppression, can avoid or substantially delay joint damage and deformities, and can maintain a good quality of life.
Optimal management requires early diagnosis and treatment, usually with combinations of conventional disease modifying antirheumatic drugs (DMARDs). If these do not effect remission, biological DMARDs may be beneficial.
Lack of recognition of the early signs of rheumatoid arthritis, ignorance of the benefits of early application of modern treatment regimens, and avoidable delays in securing specialist appointments may hinder achievement of best outcomes for many patients.
Triage for recognising possible early rheumatoid arthritis must begin in primary care settings with the following pattern of presentation as a guide:
involvement of three or more joints;
early-morning joint stiffness of greater than 30 minutes; or
bilateral squeeze tenderness at metacarpophalangeal or metatarsophalangeal joints.