How and to what extent do companion animals confer health benefits on people? We know that compared with people without a pet, people who keep a pet have been reported to adapt more quickly to stress associated with bereavement and other adverse events,1,2 require fewer visits to the doctor,3 and have stronger emotional stability and maintain a generally sounder state of health.2 In experiments in which older men and women living alone were randomly selected to receive either a cage bird or a potted plant (begonia), those receiving a budgerigar exhibited health benefits from the outset of keeping the bird.4 Similarly, keeping either a dog or cat has been shown to alleviate minor physical and emotional problems as early as the first month of caring for the pet.5
Specific human clinicophysiological benefits of companion animals were initially reported a quarter of a century ago, when a relationship between pet ownership and higher survival rates 1 year after hospital admission for coronary heart disease was documented.6 Bonding with a companion animal was credited with leading to beneficial effects on the human cardiovascular system.7 In patients with hypertension who were under stress, patting and talking to a pet dog or cat may have prevented increased blood pressure.8 In a study investigating risk factors for cardiovascular disease, it was found that dog owners had lower systolic blood pressure and lower levels of serum triglycerides than non-dog owners.9
Despite our knowledge of these unequivocal enhancements in human health and physiology associated with close contact with companion animals, the mechanisms for these beneficial effects remain elusive, and are, therefore, worthy of new and robust research. Higher concentrations of high-density lipoprotein (HDL) cholesterol have been attributed to the increased daily physical activity inherent in pet ownership, for example, dog walking.5
While health gains from walking are well established, it is not known whether walking a dog provides greater benefit than walking without a dog. Moreover, we do not know whether the physiological benefits of interacting with a companion animal would be ruled out for people unable to walk. As variability in the autonomic nervous system plays a crucial role in almost every vital function,10 analysing the oscillations of parasympathetic neural activity, equated with cardiac vagal tone, would conceivably shed light on the physiological effects a pet dog might have on a person walking the dog or patting the dog. We hypothesised that in senior citizens, walking a dog provides greater health benefits than walking without a dog, and that the difference would be reflected by an increase in parasympathetic neural activity, which is generally associated with a reduction in stress.
We used a crossover experimental design in which each participant served as his or her own control. Volunteers were recruited from 1 September 2003 to 1 March 2004, and the experiments were conducted in March and April 2004 (springtime in Japan). Participants were 60 years old or older and deemed by their physician to be in good health. In total, 13 volunteers (three men, 10 women) ranging from 62 to 82 years of age (mean 67.5 years) participated in the dog-walking study, and all gave their informed consent in writing. In addition, four of the volunteers gave written consent to participate in a 6-hour related study in their respective homes.
Heart-rate variability analysis: Heart-rate variability (HRV) has attracted increasing interest as a non-invasive surrogate index of autonomic nervous activity10-15 Given that the underlying physiology of circulation is regulated by the autonomic nervous system and that rhythms of the RR interval are mediated largely by fluctuations of vagal–cardiac nerve activity, we developed a non-invasive research design based on spectral analysis of HRV. Numerous methods have been used to measure HRV.10,16,17 In the frequency domain, non-random components of the RR interval are assessed by spectral techniques based on the fast Fourier transform algorithm, and high frequency (HF) power is the major component in the power spectrum that has metric utility.16,17 Our research model provided constant monitoring of HRV, including extended periods when participants were walking a dog.
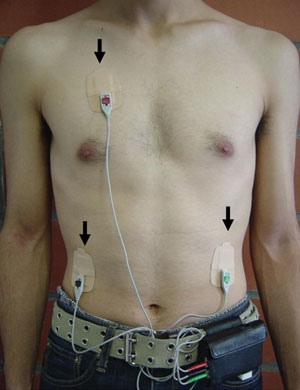
A palm-sized electrocardiograph (ECG) unit (Active Tracer, GMS, Tokyo, Japan) was used for continuous monitoring of variation in participants’ autonomic nervous activity, as computed from changes in the RR interval. The ECG unit (7 cm high × 7.7 cm wide × 1.5 cm deep, weighing 87 g) was worn in a pouch at the waist with three electrodes taped to the chest (Box 1). When variability of the RR interval was obtained, the maximum entropy method was automatically applied to analyse the power spectrum. HF power was calculated from the RR values in designated 60-second intervals, and used as the index for parasympathetic neural activity. The validity of analysing HRV to assess autonomic nervous function has been verified in studies using autonomic nerve blocking agents;10,18,19 spectral analysis of the RR interval cannot be used in patients with atrial fibrillation, atrial flutter, or ventricular fibrillation.
Walking with and without a dog: We analysed human autonomic nervous activity for parasympathetic oscillation while the volunteer was walking without a dog and walking a dog. We focused on the effects of the presence or absence of a dog, not on ownership of a dog. Thus, we used a single friendly dog that the participants had not previously encountered (a 2-year-old Cavalier King Charles Spaniel intact bitch) in all experiments (Box 2). We chose a dog for this study because in earlier work in which patients who had survived acute myocardial infarction owned a cat, rabbit, horse, fish or other pet, dogs were found to have the greatest impact.20
Participants’ blood pressure was measured just before starting the experiment. Each participant was fitted with the ECG unit and required to take two 30-minute walks outdoors in the park adjacent to Gunma University Medical Hospital. Participants were randomly assigned to either walk the dog first or walk without the dog first. Participants thus walked for 30 minutes, rested for 20 minutes (to allow for complete stabilisation of the heart-rate interval, which we found required 15 minutes in preliminary experiments) and then walked for a further 30 minutes.
We asked for volunteers among the participants to continue the walking program for 3 consecutive days to enable individual crossover between walking the dog first and walking without the dog first.
Interacting with the dog at home: We also requested volunteers from among the original participants for continuous 6-hour monitoring during routine activities in their homes. In two 30-minute intervals during the 6 hours, these volunteers were allowed to spend time interacting spontaneously with the dog.
Thirteen people agreed to participate in the dog-walking study (3 men, 10 women; age range, 62–82 years; mean age, 67.5 years); all gave written informed consent. After completing the 80-minute walking program, a subgroup of three of the original participants (1 man, 2 women; mean age, 63.3 years) gave written consent to continue the walking program for 3 consecutive days to enable us to complete the crossover component of our study. Four participants (all women; mean age, 71.0 years) also gave written consent to undergo continuous 6-hour monitoring during routine activities and periods of interaction with the dog in their homes.
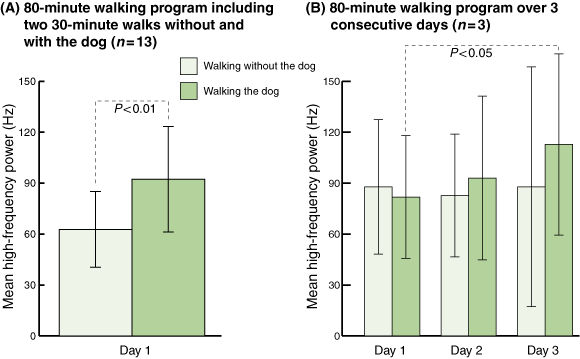
Six participants walked without the dog first, then rested for 20 minutes, then walked the dog. The other seven walked the dog first, rested, then walked without the dog. When participants were walking without the dog, they had a mean HF power of 62.76 Hz (SD, 22.27; 95% confidence interval [CI], 40.49–85.03; Box 3A). In contrast, when participants were walking the dog, the mean HF power rose significantly to 92.28 Hz (SD, 31.10; 95% CI, 61.18–123.39; P < 0.01).
In the extended 3-day walking program, when participants were walking without the dog, their mean HF power was 87.84 Hz (SD, 39.61; 95% CI, 48.23–127.45) on Day 1, 82.67 Hz (SD, 36.17; 95% CI, 46.51–118.84) on Day 2; and 87.84 Hz (SD, 70.61; 95% CI, 17.23–158.45) on Day 3 (Box 3B). When participants were walking the dog their mean HF power rose steadily from 81.81 Hz (SD, 36.17; 95% CI, 45.64–117.98) on Day 1 to 93.00 Hz (SD, 48.22; 95% CI, 44.76–141.20) on Day 2, and to 112.81 Hz (SD, 53.39; 95% CI, 59.42–166.20) on Day 3. Compared with Day-1 measurements, the dog-walking HF power values for Day 3 showed a significant increase (P < 0.05). The order of doing the experiments, whether walking the dog first or walking without the dog first, made no difference.
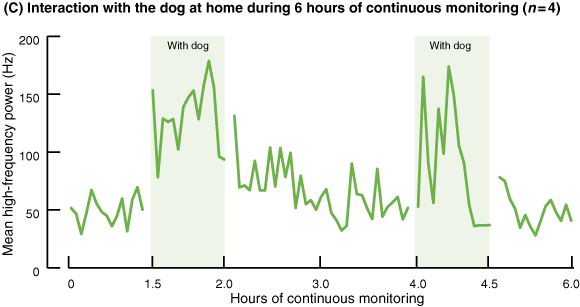
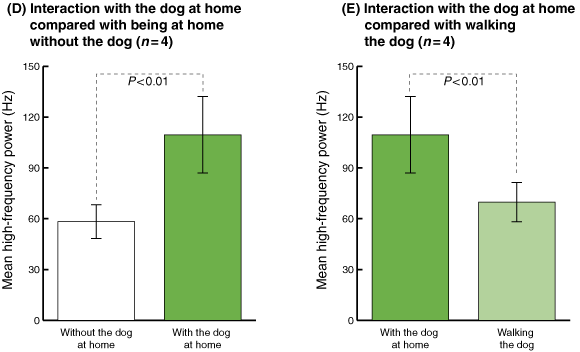
During periods without the dog, the average HF power was 58.33 Hz (SD, 9.93; 95% CI, 48.40–68.25) (Box 3C and D). However, in the presence of the dog, average HF power values increased by 1.87 times (109.54 Hz; SD, 22.54; 95% CI, 87.00–132.08). HF power was consistently significantly greater when the dog was present than when it was not (P < 0.01).
Moreover, in the presence of the dog at home, the HF power was 1.57 times higher (P < 0.01) than when these participants were walking the dog (Box 3E).
Our findings support our dual hypothesis: that in senior citizens:
walking a dog provides potentially greater health benefits than walking without a dog; and
walking a dog shifts a person’s autonomic nervous activity in favour of parasympath-etic activity.
In addition, the augmented parasympathetic pattern on successive dog walks shows that the magnitude of change in cardiac vagal tone can possibly be heightened by frequent exposure to a friendly dog.
Further, we found that, not only did parasympathetic neural activity predominate throughout the dog’s visit at the participant’s home, but also that the magnitude of increase far exceeded our expectations — the HF power associated with interacting with the dog at home was one-and-a-half times greater than that associated with walking the dog. We observed that host participants lavished praise and attention on the dog throughout its visits — rubbing, patting, talking to it, and touching the dog almost the whole time. It may be instructive, therefore, to re-examine the supposition that health benefits to humans occur because pet owners are forced to increase their daily physical activity by such means as dog walking.5 We believe that it is not the exercise of walking that suppresses sympathetic nervous activity, but rather being in the presence of the dog, or involving oneself with the dog, that brings about the parasympathetic surge. It has been reported that continually talking to a dog stabilises a patient’s heart rate.8 Our findings support the argument that, even for people unable to walk a dog, merely spending time with a companion dog may have a stabilising effect. It has been surmised that pet factors in protecting physical health depend on the owner’s level of attachment to the animal.2 However, our results show that an unknown, friendly dog can elicit a positive and measurable effect on the human physiological condition.
Humans interact with dogs by using all five senses, especially sight and touch. A wide range of visual and tactile information is projected afferently to the cerebrum via the optic and other sensory nerves, and the cerebral information is directed into the autonomic nervous system. This, in all likelihood, prompts the pet-induced surge in parasympathetic neural activity. Plausibly, pet companionship buffers human stress,21 thus increasing the aroused parasympathetic neural activity, and the enhancement of this component in the rhythmic spectrum is the likely mechanism that helps keep health problems at bay.
In view of our results, previously reported findings1-9,20 may be explained in part by the rhythmic shift in autonomic nervous activity in favour of the parasympathetic. Effort aimed at modifying autonomic nervous activity is currently a therapeutic focus, not only for the heart, but also for other organs. Stress-related sympathetic nervous activity, for example, is known to inhibit progenitor cell accumulation in damaged liver, but it has been found that pharmaceutical blockade of sympathetic nervous activity can enhance hepatic progenitor cell accumulation, promoting liver regeneration.22 Little is known about the mediators that regulate repopulation of oval cells, the resident hepatic stem cells that promote liver repair.22 We propose that a friendly companion dog might help promote parasympathetic neural activity in patients with an injured liver, and this could be particularly helpful in those with low tolerance for long-term use of certain pharmaceutical agents.
Potential specific applications aside, our study bears out that in senior citizens, walking a dog facilitates potentially greater health benefits than walking without a dog and provides a compelling argument that the dog-induced upsurge in parasympathetic neural activity is the mechanism behind acclaimed human health benefits associated with companion animals. Further, the noninvasive power spectral analysis of HRV used in this study may be useful in further clinical research.
Received 2 August 2005, accepted 1 November 2005
- Masahiko Motooka1
- Nell L Kennedy2
- Hiroto Koike3
- Tomoyuki Yokoyama4
- 1 School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Hokkaido, Japan.
- 2 School of Health Science, Gunma University, Maebashi, Gunma, Japan.
We are grateful to the Companion Animal Research Center, Tokyo, for support in this research. We are also indebted to Harumi Motooka, registered nurse, for assistance during the experiments; Dr Hiroki Inoue for technical assistance; and Dr Takio Kitazawa for statistical advice.
None identified.
- 1. Akiyama H, Holtzman JM, Britz WE. Pet ownership and health status during bereavement. Omega J Death Dying 1987; 17: 187-193.
- 2. Garrity TF, Stallones L, Marx MB, Johnson TP. Pet ownership and attachment as supportive factors in the health of the elderly. Anthrozoos 1989; 3: 35-44.
- 3. Siegel JM. Stressful life events and use of physician services among the elderly: the moderating role of pet ownership. J Pers Soc Psychol 1990; 58: 1081-1086.
- 4. Mugford RS, M’Cornisky JG. Therapeutic value of cage birds with old people. In: Anderson ER, editor. Pet animals and society. London: Balliere Tindall; 1975: 54-65.
- 5. Serpell JA. Beneficial effects of pet ownership on some aspects of human health. J Roy Soc Med 1991; 84: 717-720.
- 6. Friedmann E, Katcher AH, Lynch JJ, Thomas SA. Animal companions and one year survival of patients after discharge from a coronary care unit. Public Health Rep 1980; 95: 307-312.
- 7. Baun MM, Bergstrom N, Langston N, Thoma L. Physiological effects of human/companion animal bonding. Nurs Res 1984; 33: 126-129.
- 8. Katcher AH. Interactions between people and their pets: form and function. In: Fogle B, editor. Interrelationships between people and pets. Springfield, Ill: Charles C Thomas; 1981: 41-67.
- 9. Anderson W, Reid P, Jennings GL. Pet ownership and risk factors for cardiovascular disease. Med J Aust 1992; 157: 298-301.
- 10. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991; 84: 482-492.
- 11. Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986; 59: 178-193.
- 12. Akita M, Ishii K, Kuwahara M, Tsubone H. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in guinea pigs. Exp Anim 2002; 51: 1-7.
- 13. Hagiwara Y, Tsubone H, Kuwahara M. Effects of endotoxin on cardiovascular and autonomic nervous system in rats. Jpn J Electrocardiol 2001; 21: 164-173.
- 14. Hashimoto M, Kuwahara, M, Tsubone H, Sugano S. Diurnal variation of autonomic nervous activity in the rat: investigation by power spectral analysis of heart rate variability. J Electrocardiol 1999; 32: 167-171.
- 15. Kuwahara M, Suzuki A, Tsutsumi H, et al. Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in miniature swine. Lab Anim Sci 1999; 49: 202-208.
- 16. Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertension 1999; 17: 719-734.
- 17. Kingwell BA, Thompson JM, Kaye DM, et al. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation 1994; 90: 234-240.
- 18. Kuwahara M, Yayou K, Ishii K, et al. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol 1994; 27: 333-337.
- 19. Kuwahara M, Hashimoto S, Tsubone H, Sugano S. Developmental changes of autonomic nervous activity in spontaneously hypertensive rats: investigation by power spectral analysis of heart rate variability. J Ambul Monit 1996; 9: 51-58.
- 20. Friedmann E, Thomas SA. Pet ownership, social support, and one-year survival after acute myocardial infarction in the cardiac arrhythmia suppression trial (CAST). Am J Cardiol 1995; 76: 1213-1217.
- 21. Friedmann E, Katcher A, Thomas SA, et al. Social interaction and blood pressure: influence of animal companions. J Nerv Ment Dis 1983; 171: 461-465.
- 22. Oben JA, Roskams T, Yang S, et al. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology 2003; 38: 664-673.







Abstract
Objective: To compare changes in autonomic nervous activity in healthy senior individuals while walking with and without a dog, and during routine activities at home and periods of interacting with the dog at home.
Design: Controlled crossover study.
Participants and setting: 13 healthy volunteers (3 men, 10 women; mean age, 67.5 years) who walked in a park adjacent to Gunma University, Japan, and 4 volunteers among these who underwent monitoring in their own homes.
Interventions: Heart rate variability was monitored continuously by means of a palm-sized electrocardiographic monitor (which facilitated spectral analysis of the RR interval) while participants walked for 30 minutes (first with, then without, the study dog, or vice versa); three participants underwent this intervention on 3 consecutive days. Four participants underwent continuous monitoring for 6 hours in their own homes, including two 30-minute periods of free interaction with the study dog.
Main outcome measures: High frequency (HF) power values of heart rate variability, which is a measure of parasympathetic neural activity.
Results: During dog-walking, HF power increased significantly (P < 0.01); this increase was sustained throughout each dog walk, and was more pronounced during succeeding dog walks. At home, HF power was 1.87 times greater when the dog was present, and 1.57 times greater (P < 0.01) than in the walking experiment.
Conclusions: Walking a dog has potentially greater health benefits as a buffer against stress in senior citizens than walking without a dog; and, independent of actually walking, merely patting and talking to a dog also raises parasympathetic neural activity. Power spectral analysis of heart rate variability shows promise as a non-invasive approach to quantifying clinicophysiological research on human health benefits possibly derived from interaction with companion animals.