Clinical records
Patient 1 A 30-year-old woman presented to hospital with bilateral hand paraesthesia and a serum potassium level of 2.9 mmol/L (reference range [RR], 3.5–5.0 mmol/L). She denied taking medications and had no significant past medical history. However, 3 months before admission, she had presented to her general practitioner complaining of anorexia, nausea, lethargy, fevers and aches. An erythrocyte sedimentation rate (ESR) of 91 mm/h (RR, 7–18 mm/h) and a serum creatinine level of 0.106 mmol/L (RR, 0.030–0.110 mmol/L) were noted. (Twelve months previously, her serum creatinine level had been 0.073 mmol/L.) Then, 1 month before admission, she had developed blurred vision due to anterior uveitis, diagnosed by an ophthalmologist, and was treated with topical steroids. In the intervening period, she had lost 12 kg in weight. A normochromic, normocytic anaemia was now present (haemoglobin level, 103 g/L; RR, 115–165 g/L), and her serum creatinine level was 0.230 mmol/L. The hypokalaemia was corrected, and she was discharged with a referral to the renal outpatient clinic. In clinic, her blood pressure was 140/85 mmHg, but the physical examination was otherwise normal. Repeat laboratory tests showed: creatinine, 0.250 mmol/L; potassium, 3.1 mmol/L; bicarbonate, 17 mmol/L (RR, 23–31 mmol/L); and phosphate, 0.64 mmol/L (RR, 0.60–1.40 mmol/L). Urine microscopy showed no leukocytes, erythrocytes or casts. Urine pH was 7.0, with glucosuria on dipstick. Protein excretion was 0.9 g/day (RR, < 0.15 g/day). Autoimmune markers were negative, including antinuclear antibody, antineutrophil cytoplasmic antibody, antidouble-stranded DNA antibody, and antibodies to extractable nuclear antigens. Serum calcium and angiotensin-converting enzyme (ACE) levels were normal. A chest x-ray and renal ultrasound were unremarkable. A renal biopsy showed acute interstitial nephritis and chronic renal damage (Figure A). After taking prednisolone 60 mg/day and concurrent phosphate, bicarbonate and potassium supplements for 4 weeks, her serum creatinine level fell to 0.130 mmol/L. The dose of steroids was reduced |
gradually over 4 months and renal function remained stable. Shortly after stopping prednisolone, her eye symptoms recurred. Ophthalmological examination revealed a bilateral visual acuity of 6/4, keratic precipitates, posterior synechiae and perilimbal injection, consistent with anterior uveitis (Figure B). This responded to topical steroids. Patient 2 A 34-year-old woman was referred to the renal clinic by her general practitioner, with a creatinine level of 0.190 mmol/L, normochromic, normocytic anaemia (haemoglobin level, 110 g/L), and an elevated ESR (120 mm/h). She gave a 1-month history of weight loss (15 kg), with anorexia, nausea, arthralgias, myalgias, fatigue and fevers. She had mild asthma, which was being treated with salbutamol, and took no other medications. Physical examination was unremarkable. Repeat laboratory tests 1 week later showed a serum creatinine level of 0.250 mmol/L. Urine microscopy showed no leukocytes, erythrocytes or casts. Proteinuria was absent and a renal ultrasound gave normal results. A renal biopsy revealed granulomatous acute interstitial nephritis with multinucleated giant cells (Figure C). Her serum calcium and ACE levels were normal, and a chest x-ray was unremarkable. Oral prednisolone 60 mg/day was commenced. One week later, her constitutional symptoms and renal function markedly improved. Repeat tests showed: creatinine, 0.120 mmol/L; potassium, 2.9 mmol/L; and phosphate, 0.40 mmol/L. Glucosuria was detected on urine dipstick analysis. All autoimmune markers and HLA-B27 were negative. Potassium supplementation was commenced. After the prednisolone dose was reduced to 2.5 mg/day over 2 months, she developed bilateral red and painful eyes, photophobia and watery discharge. Ophthalmological examination showed bilateral anterior uveitis, with a visual acuity of 6/9 bilaterally, perilimbal injection, keratic precipitates, anterior chamber cells 3+, and posterior synechiae. The uveitis resolved with topical steroids and cycloplegic agents. Her serum creatinine level remained stable without steroid dose adjustment. |
||||||||||||||
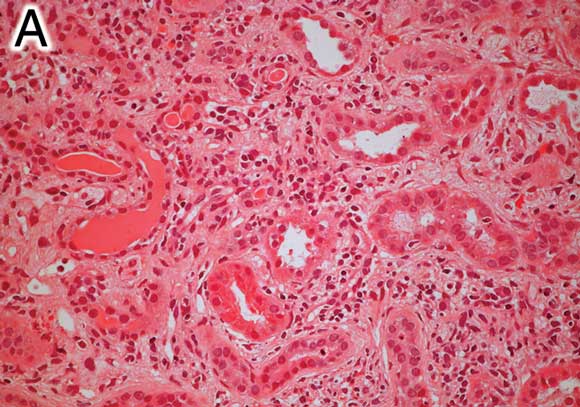 A: Renal biopsy specimen (Patient 1) showing an interstitial infiltrate of lymphocytes, plasma cells, histiocytes and eosinophils, without granulomas. There is prominent tubular atrophy and wide separation of tubular structures due to interstitial fibrosis (haematoxylin–eosin stain, original magnification x 200). |
|||||||||||||||
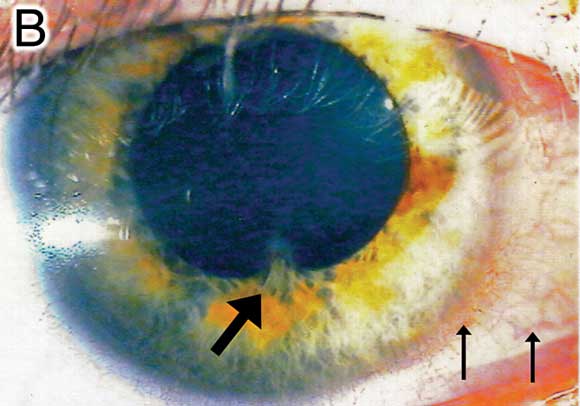 B: Photograph of the right eye of Patient 1, with the thick arrow showing pupil irregularity and posterior synechiae, and thin arrows showing inflammatory perilimbal injection. Keratic precipitates and anterior chamber cells are best appreciated on slit lamp examination. |
|||||||||||||||
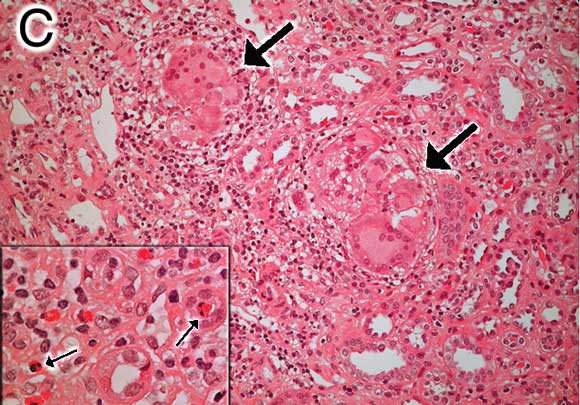 C: Renal biopsy specimen (Patient 2) showing tubulointerstitial mononuclear infiltrate with non-caseating granulomas and multinucleated giant cells (large arrows) (haematoxylin–eosin stain, original magnification x 200). Inset: Small arrows highlight eosinophils with typical bilobed nuclei (x 400). |
|||||||||||||||
Tubulointerstitial nephritis and uveitis syndrome (TINU) was first reported in 1975.1 Diagnosis of TINU requires identification of acute interstitial nephritis and uveitis, in the absence of systemic diseases associated with either condition. TINU occurs more frequently in females (3 : 1), with the median age of onset being 15 years, and has no racial association.2 At least 50% of cases are probably idiopathic based on the absence of risk factors for acute interstitial nephritis.2 Associations that have been reported include: drugs (antibiotics, non-steroidal anti-inflammatory drugs), infections (herpes zoster, Epstein–Barr virus, toxoplasmosis), and systemic diseases (hyperthyroidism, hypoparathyroidism, rheumatoid arthritis).2 The main differential diagnosis is sarcoidosis. Although uveitis associated with sarcoidosis is typically granulomatous, uveitis associated with TINU is mostly non-granulomatous. Sarcoidosis rarely causes acute interstitial nephritis and frequently affects the lungs, whereas lung involvement has not been reported with TINU. Sjögren’s syndrome is not a differential diagnosis because patients with Sjögren’s syndrome do not develop uveitis despite having sore eyes (sicca). Neither patient was taking medications known to cause acute interstitial nephritis.
There is often a time interval between the diagnosis of uveitis and that of acute interstitial nephritis, making the diagnosis of TINU difficult. In 35% of patients with TINU, ocular findings precede or develop concurrently with acute interstitial nephritis. In 65% of patients, ocular symptoms follow acute interstitial nephritis by a median time of 1 month, but can occur up to 14 months later.2 The most common systemic features are fever, weight loss, fatigue and malaise (50%); and eye pain and redness are the most usual ocular symptoms (77%).2 Acute anterior uveitis is the typical finding (80%) and is usually bilateral. About 20% of patients develop ocular complications, such as posterior synechiae (most common), cataracts and glaucoma.
Patient 1 developed recurrence of uveitis despite quiescent renal disease, showing that the course of ocular disease can be independent of renal disease.2,3 Uveitis recurs or becomes chronic in about 50% of patients. It is commonly treated with topical or systemic steroids, and cycloplegic agents. Methotrexate, cyclosporin or azathioprine may prevent relapses in steroid-resistant, recurrent or persistent uveitis, but randomised trials are lacking.2,4
Both patients demonstrated features of proximal tubular dysfunction consistent with Fanconi’s syndrome. This syndrome causes aminoaciduria, glucosuria, metabolic acidosis (bicarbonate wasting), hypophosphataemia, natriuresis, kaliuresis, polyuria and proteinuria. It has been reported in idiopathic interstitial nephritis,3 drug-related interstitial nephritis,5 and TINU.6,7 Incomplete Fanconi’s syndrome and distal tubular defects with hyperkalaemia have also been reported.8 Urinary electrolyte losses can be significant and symptomatic, as in Patient 1.
Another complication that has been found is chronic renal damage. Some authors consider renal disease in TINU to be benign,9 but the renal biopsy from Patient 1 suggests that TINU produces chronic damage. Renal failure may resolve spontan-eously and almost always responds to steroids.2 Persistent renal dysfunction occurs in about 10% of patients, with few needing dialysis. Renal biopsy findings are typical of acute interstitial nephritis, with eosinophils seen in 34% and non-caseating granulomas in 13%.2 Granulomas have been described in lymph nodes and bone marrow.1
Despite its propensity to affect the young, TINU is not limited to paediatric patients, as these cases demonstrate. TINU may be underreported, given the frequent temporal dissociation between uveitis and acute interstitial nephritis. Chronic renal damage and electrolyte abnormalities do occur; hence, patients with uveitis should be evaluated for renal involvement. If any abnormalities are detected, a renal biopsy may be indicated. Early detection and steroid treatment may prevent renal complications.
- 1. Dobrin RS, Vernier RL, Fish AJ. Acute eosinophilic interstitial nephritis and renal failure with bone marrow–lymph node granulomas and anterior uveitis. A new syndrome. Am J Med 1975; 59: 325-333.
- 2. Mandeville JTH, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol 2001; 46: 195-208.
- 3. Wakaki H, Sakamoto H, Awazu M. Tubulointerstitial nephritis and uveitis syndrome with autoantibody directed to renal tubular cells. Pediatrics 2001; 107: 1443-1446.
- 4. Takemura T, Okada M, Hino S, et al. Course and outcome of tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis 1999; 34: 1016-1021.
- 5. Spital A, Panner BJ, Sterns RH. Acute idiopathic tubulointerstitial nephritis: report of two cases and review of the literature. Am J Kidney Dis 1987; 9: 71-78.
- 6. Neelakantappa K, Gallo GR, Lowenstein J. Ranitidine-associated interstitial nephritis and Fanconi syndrome. Am J Kidney Dis 1993; 22: 333-336.
- 7. Igarashi T, Kawato H, Kamoshita S, et al. Acute tubulointerstitial nephritis with uveitis syndrome presenting as multiple tubular dysfunction including Fanconi’s syndrome. Pediatr Nephrol 1992; 6: 547-549.
- 8. Braden GL, Germain MJ, Fitzgibbons JP. Impaired potassium and magnesium homeostasis in acute tubulo-interstitial nephritis. Nephron 1985; 41: 273-278.
- 9. Gion N, Stavrou P, Foster CS. Immunomodulatory therapy for chronic tubulointerstitial nephritis-associated uveitis. Am J Ophthalmol 2000; 129: 764-768.





We thank Dr Sarah Parsons from the Department of Anatomical Pathology, Austin Health, for providing the renal histology for both patients.
None identified.