Worldwide, the incidence of childhood type 1 diabetes mellitus (T1DM) varies widely — there is a more than 350-fold difference between annual rates reported in Finland (36.5/100 000) compared with those in China (0.1/100 000).1 However, epidemiological studies have shown a high, and rising, incidence of childhood T1DM in the past two decades in Europe2-5 and Australia.6,7 The incidence of T1DM in New South Wales in children aged 0–14 years increased on average by 3.2% per year from 1990–1996, with a doubling of the incidence in children under 5 years of age.6 This is consistent with several European studies in which the incidence of T1DM increased predominantly in the younger age groups.2,3 In contrast, in a recently reported study from Western Australia covering the period 1985–2002, the incidence more than doubled, but there was not a disproportionate increase in younger children.7
The incidence of type 2 diabetes is also increasing in childhood,8-10 and parallels the significant rise in childhood obesity in many Western countries including Australia.11,12 It is possible that the obesity epidemic is also a factor in the increased incidence of T1DM, as it is widely accepted that T1DM develops as a result of environmental trigger(s) in genetically predisposed individuals. In early childhood, rapid growth in height and obesity are risk factors for developing T1DM,13 and the “accelerator hypothesis” predicts earlier onset of T1DM in heavier children.14 In line with the increasing rates of childhood obesity in Australia,11,12 ongoing increases in incidence of childhood T1DM and earlier onset of disease may also be expected in NSW.
Our aim was to determine the incidence of T1DM in NSW for 1997–2002, evaluate whether the previously described rise in incidence in 1990–1996 has continued,6 and analyse epidemiological trends in age and sex of children with T1DM over the entire period (1990–2002).
Incident cases of T1DM have been ascertained prospectively from 1990 by the Australasian Paediatric Endocrine Group (APEG) NSW children’s diabetes register, as previously described.6,15 Secondary ascertainment was from the National Diabetes Supply Scheme until 1999, and from the Australian Institute of Health and Welfare from 2000. The capture–recapture method was used to determine completeness of ascertainment, and data from all ascertainment sources were included in the analyses.16 Childhood population estimates were based on census data obtained from the Australian Bureau of Statistics.17
Primary ascertainment by the APEG register found 93% of subjects. Overall ascertainment using the two independent sources and the capture–recapture method was estimated to be 99.7% complete for the entire study period.16 Based on this estimate, it is expected that nine patients with T1DM were missed by both sources of ascertainment over the 13 years (1990–2002).
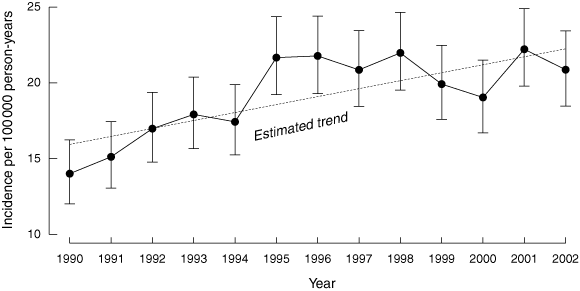
From 1990 to 2002 inclusive, 3260 children were diagnosed with T1DM (1629 boys and 1631 girls). The mean annual incidence over the 13-year study period was 19.3 per 100 000 person-years (95% CI, 18.6 to 19.9). The lowest annual incidence was 14 per 100 000 (95% CI, 12.0 to 16.2) in 1990, and the incidence peaked at 22.2 per 100 000 (95% CI, 19.8 to 24.7) in 2001 (Box 1).
The age-standardised incidence for all children aged 0–14 years during the two study periods (1990–1996 and 1997–2002) is shown in Box 2; the incidence increased by 16.4% between the two study periods. There was a plateau from 1997 to 2002, with no significant increase in incidence over this time; however, for the 13-year study period the incidence increased on average by 2.8% per year (rate ratio, 0.028; 95% CI, 0.019 to 0.038), after adjusting for age group and sex (Box 2).
The mean annual incidence for the 13-year study period in the three age groups was 12.2 per 100 000 (95% CI, 11.3 to 13.1) in 0–4 year olds, 18.9 per 100 000 (95% CI, 17.8 to 20.0) in 5–9 year olds, and 26.7 per 100 000 (95% CI, 25.4 to 28.1) in 10–14 year olds. The peak annual incidence was 38.0 per 100 000 (95% CI, 30.5 to 46.9) in boys 10–14 years in 2001. Compared with the youngest age group, the incidence was significantly higher in 5–9 year olds (rate ratio, 1.50; 95% CI, 1.31 to 1.72) and 10–14 year olds (rate ratio, 2.36; 95% CI, 2.08 to 2.68).
The incidence of T1DM by age group and sex over the two study periods, and average annual increase per year, are shown in Box 2. The incidence increased in all age groups, and the rise in 0–4 year olds (3.9%) was not significantly higher than in the other age groups. The mean age (SD) of onset was unchanged between the two study periods: 8.9 (3.9) years from 1990–1996 and 8.8 (4.0) years from 1997–2002 (P = 0.8).
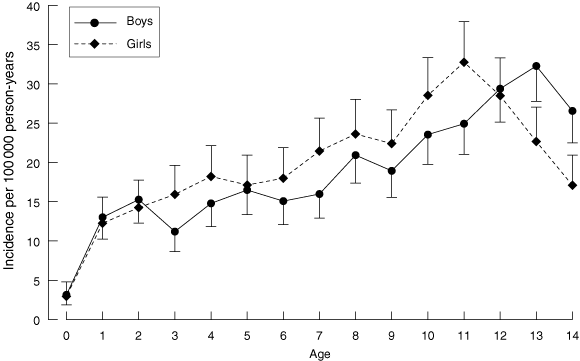
The mean annual incidence for the 13-year study period was 18.8 per 100 000 (95% CI, 17.9 to 19.7) in boys and 19.8 (95% CI, 18.8 to 20.7) in girls. After adjusting for age group and year, the incidence was significantly higher in girls (P = 0.02) over the entire study period, but the difference was not significant (P = 0.6) comparing girls with boys in the second study period alone. The incidence increased in both sexes (Box 2), and the average annual increase was significantly higher in boys (3.8% v 1.9%, P = 0.046). There was no difference in the average increase in boys compared with girls when the two study periods were analysed separately. Mean annual incidence varied with age in both sexes and peaked at 32.8 per 100 000 (95% CI, 28.2 to 37.9) in girls aged 11 years and 32.3 per 100 000 (95% CI, 27.8 to 37.3) in boys aged 13 years (Box 3). Mean age (SD) of onset of diabetes was earlier in girls: 8.7 (3.8) years compared with 9.1 (4.1) years in boys (P = 0.005).
The incidence of childhood T1DM has increased significantly in NSW since 1990, with an apparent plateau of about 21 per 100 000 person-years since 1995. The incidence was significantly higher in girls (19.8 v 18.8 per 100 000) and peaked in girls earlier than in boys (11 v 13 years); however, no sex differences were found in the second study period. The incidence increased significantly in both sexes and in all age groups, without a disproportionate rise in children aged 0–4 years. The incidence was significantly higher in the age groups 5–9 and 10–14 years, compared with the youngest age group.
The accuracy of incidence estimates from disease registers may be affected by incomplete ascertainment or misclassification, resulting in under- or overestimation of incidence. In our study, ascertainment was over 99% complete, indicating that very few cases would have been missed by the two independent sources of ascertainment. The completeness of ascertainment has not changed since the establishment of the NSW register in 1990;6,15 it is in keeping with previous reports from WA,7 and it fulfils recommendations for diabetes incidence registers as part of the World Health Organization Multinational Childhood Diabetes (DiaMond) Project.18 It is also unlikely that misclassification contributed to the observed increase in incidence. The diagnosis of T1DM in the APEG NSW diabetes register and the National Diabetes Supply Scheme is certified by a doctor or diabetes educator, minimising the possibility of misdiagnosis, and alternative diagnoses include type 2 diabetes or “other diabetes”. Furthermore, most children and adolescents with newly diagnosed diabetes in NSW are tested for diabetes-associated autoantibodies, confirming the diagnosis of T1DM.19,20 Increased recognition of cases of type 2 diabetes9 and the establishment of a register for childhood type 2 diabetes in NSW8 are also likely to have minimised misclassification bias.
The average annual increase of 2.8% per year is less than that reported in NSW from 1990–1996,6 and less than that in WA where incidence increased by 3.1% per year from 1985–2002.7 The increase in incidence was significant only in the first half of the 1990s. It is possible, however, that the more recent plateau in incidence represents a temporal variation in incidence and that the change in incidence over the 13-year period gives a better indication of longer term trends. Indeed, epidemiological data from populations with a similar overall incidence to NSW, and study periods as long as, or longer than, that reported here demonstrate a consistently rising incidence of T1DM,2,7,21 suggesting that the NSW trend will continue.
Based on arbitrarily defined groupings used to analyse global incidence of T1DM,1 the incidence in NSW would now be classified as “very high”, along with other populations with previously published annual incidences of at least 20 per 100 000 including those in WA, Finland, Sardinia, Canada, Norway, Aberdeen (United Kingdom) and Canterbury (New Zealand).1,22,23
The female sex bias in T1DM incidence in NSW, apparent in the first half of the 1990s, was not sustained in the second study period. In the recent report from WA (1985–2002), there was a trend for a higher incidence in girls (17.3 v 15.6 per 100 000), which did not reach statistical significance (P = 0.1).7 A trend to higher T1DM incidence in girls has been reported in populations with low rates of T1DM, such as South America, Africa and southern Europe, while a male excess has been reported in many European populations with a high incidence.1,24 We speculate that the absence of a significant male sex bias in the incidence of T1DM in NSW may be related to the ethnic diversity of the NSW population, associated with a lower rate of high-risk HLA genotypes, in comparison with European countries, and a subtype of virus-induced T1DM.20
The highest rates of T1DM were found in the 10–14 year age group, in line with other published studies.4 The average rise in annual incidence appeared to be higher in the youngest age group (3.9% per year in 0–4 year olds), although this did not reach statistical significance. In contrast to recent reports of a greater rise in the youngest age group and a significant lowering in the age of diabetes onset,3,5 the increase in NSW has been uniform across all ages, as was the case in WA over a similar time period.7
These trends in diabetes incidence are more likely to be related to environmental factors rather than changes in the population prevalence of genetic susceptibility, because the increase has occurred over a relatively short period of time.21 Enteroviruses have been associated with the onset of T1DM20,25 and pre-diabetes,26,27 and reduced maternal immunity to enteroviruses has been suggested as a cause of the rising incidence.28 In many of the populations with the highest incidence of T1DM, childhood overweight and obesity are also on the rise, and the prevalence of overweight is increasing in children at onset of T1DM.29 Higher rates of insulin resistance in children may lead to β-cell fatigue and destruction, the end-point being increasing rates of childhood T1DM. Although these are only associations and do not prove causality, it is possible that these trends are linked.
The rising incidence of childhood diabetes in NSW, in keeping with international data, suggests new or increasing exposure to certain triggers in at-risk populations. Prospective studies in Australia investigating the role of cow’s milk protein and viruses as early triggers of β-cell autoimmunity may provide valuable insight into the effect of environmental factors on diabetes incidence.
Identifying incidence trends allows appropriate resource planning for the ongoing needs of these children and their families. The ongoing collection of epidemiological data in NSW and throughout Australia will demonstrate whether the recently observed plateau is a short-term temporal variation or a true stabilisation in the incidence of childhood diabetes.
2 Mean annual age and sex-specific incidence of childhood type 1 diabetes in New South Wales and percentage increase per year
|
Mean annual incidence per 100 000 person-years (95% CI)
|
Percentage increase between the two study periods (based on rate ratio; 95% CI) |
Mean percentage increase per year 1990–2002 (based on rate ratio; 95% CI) |
||||||||||||
Period |
|||||||||||||||
Boys |
|
|
|
|
|||||||||||
0–4 years |
10.3 (8.7 to 12.0) |
13.1 (11.2 to 15.2) |
27.7 (3.0 to 58.4) |
4.6 (1.6 to 7.7) |
|||||||||||
5–9 years |
15.8 (13.9 to 17.9) |
19.2 (17.0 to 21.7) |
21.5 (2.1 to 44.6) |
3.7 (1.3 to 6.2) |
|||||||||||
10–14 years |
24.3 (21.8 to 26.9) |
30.9 (28.0 to 34.0) |
27.3 (10.6 to 46.4) |
3.6 (1.6 to 5.5) |
|||||||||||
Total* |
16.7 (15.6 to 18.0) |
21.1 (19.7 to 22.6) |
25.5 (13.9 to 38.4) |
3.8 (2.5 to 5.2) |
|||||||||||
Girls |
|
|
|
|
|||||||||||
0–4 years |
11.7 (10.0 to 13.5) |
14.2 (12.2 to 16.4) |
21.8 (− 1.2 to 50.1) |
3.3 (0.4 to 6.3) |
|||||||||||
5–9 years |
19.9 (17.7 to 22.4) |
21.0 (18.6 to 23.6) |
5.2 (− 10.7 to 24.0) |
1.6 (− 0.6 to 3.9) |
|||||||||||
10–14 years |
25.5 (23.0 to 28.3) |
26.5 (23.8 to 29.5) |
4.0 (− 10.2 to 20.5) |
1.4 (− 0.6 to 3.4) |
|||||||||||
Total* |
19.0 (17.7 to 20.3) |
20.6 (19.2 to 22.1) |
8.0 (− 2.0 to 19.0) |
1.9 (0.6 to 3.2) |
|||||||||||
Boys and girls |
|
|
|
|
|||||||||||
0–4 years |
10.9 (9.8 to 12.2) |
13.6 (12.3 to 15.1) |
24.6 (7.3 to 44.8) |
3.9 (1.8 to 6.0) |
|||||||||||
5–9 years |
17.8 (16.3 to 19.4) |
20.1 (18.4 to 21.8) |
12.6 (− 0.04 to 26.9) |
2.6 (1.0 to 4.3) |
|||||||||||
10–14 years |
24.9 (23.1 to 26.8) |
28.8 (26.8 to 30.9) |
15.6 (4.5 to 27.9) |
2.5 (1.1 to 3.9) |
|||||||||||
Total* |
17.8 (17.0 to 18.7) |
20.9 (19.9 to 21.9) |
16.4 (8.7 to 24.7) |
2.8 (1.9 to 3.8) |
|||||||||||
* Age-standardised incidence. |
|||||||||||||||
Received 24 January 2005, accepted 19 July 2005
- Craig E Taplin1
- Maria E Craig2
- Margaret Lloyd3
- Martin Silink4
- Neville J Howard5
- Claire Taylor6
- Patricia Crock7
- 1 Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, Westmead, NSW.
- 2 Department of Paediatrics, St George Hospital, Kogarah, NSW.
- 3 Department of Paediatric Endocrinology, John Hunter Children’s Hospital, Newcastle, NSW.
We wish to thank Louise Catanzariti from the Australian Institute of Health and Welfare for assistance with secondary ascertainment; Albert Chan for statistical advice; Dr Charles Verge; and the children with type 1 diabetes mellitus and their families.
None identified.
- 1. Karvonen M, Viik-Kajander M, Moltchanova E, et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000; 23: 1516-1526.
- 2. Karvonen M, Pitkaniemi J, Tuomilehto J. The onset age of type 1 diabetes in Finnish children has become younger. Diabetes Care 1999; 22: 1066-1070.
- 3. Schoenle EJ, Lang-Muritano M, Gschwend S, et al. Epidemiology of type 1 diabetes mellitus in Switzerland: steep rise in incidence in under 5 year old children in the past decade. Diabetologia 2001; 44: 286-289.
- 4. Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989-1998. Diabetologia 2001; 44 Suppl 3: B3-B8.
- 5. Feltbower RG, McKinney PA, Parslow RC, et al. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003; 20: 437-441.
- 6. Craig ME, Silink M, Howard NJ, Chan A. The rising incidence of childhood type 1 diabetes in New South Wales, Australia. J Pediatr Endocrinol Metab 2000; 13: 363-372.
- 7. Haynes A, Bower C, Bulsara MK, et al. Continued increase in the incidence of childhood type 1 diabetes in a population-based Australian sample (1985-2002). Diabetologia 2004; 47: 866-870.
- 8. Broyda V, Craig ME, Crock P, Howard NJ. Incidence of type 2 diabetes in children and adolescents in New South Wales and the Australian Capital Territory. Diabetes 2003; 52 (Suppl 1): A402.
- 9. McMahon SK, Haynes A, Ratnam N, et al. Increase in type 2 diabetes in children and adolescents in Western Australia. Med J Aust 2004; 180: 459-461.
- 10. American Diabetes Association. Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics 2000; 105: 671-680.
- 11. Magarey AM, Daniels LA, Boulton JC. Prevalence of overweight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard international definitions. Med J Aust 2001; 174: 561-564.
- 12. Booth ML, Wake M, Armstrong T, et al. The epidemiology of overweight and obesity among Australian children and adolescents, 1995-97. Aust N Z J Public Health 2001; 25: 162-169.
- 13. Hypponen E, Virtanen SM, Kenward MG, et al. Obesity, increased linear growth, and risk of type 1 diabetes in children. Childhood Diabetes in Finland Study Group. Diabetes Care 2000; 23: 1755-1760.
- 14. Kibirige M, Metcalf B, Renuka R, Wilkin TJ. Testing the accelerator hypothesis: the relationship between body mass and age at diagnosis of type 1 diabetes. Diabetes Care 2003; 26: 2865-2870.
- 15. Verge CF, Silink M, Howard NJ. The incidence of childhood IDDM in New South Wales, Australia. Diabetes Care 1994; 17: 693-696.
- 16. LaPorte RE, McCarty D, Bruno G, et al. Counting diabetes in the next millennium. Application of capture-recapture technology. Diabetes Care 1993; 16: 528-534.
- 17. Australian Bureau of Statistics. Estimated resident population by age and sex in statistical local areas — New South Wales. Canberra: ABS, 2003. (ABS Catalogue No. 3201.0.)
- 18. The WHO Multinational Project for Childhood Diabetes Group. Familial insulin-dependent diabetes mellitus (IDDM) epidemiology: standardization of data for the DIAMOND Project. Bull World Health Organ 1991; 69: 767-777.
- 19. Verge CF, Howard NJ, Rowley MJ, et al. Antiglutamate decarboxylase and other antibodies at the onset of childhood IDDM: a population-based study. Diabetologia 1994; 37: 1113-1120.
- 20. Craig ME, Howard NJ, Silink M, Rawlinson WD. Reduced frequency of HLA DRB1*03-DQB1*02 in children with type 1 diabetes associated with enterovirus RNA. J Infect Dis 2003; 187: 1562-1570.
- 21. Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004; 364: 1699-1700.
- 22. Casu A, Pascutto C, Bernardinelli L, Songini M. Type 1 diabetes among Sardinian children is increasing: the Sardinian Diabetes Register for Children Aged 0-14 Years (1989-1999). Diabetes Care 2004; 27: 1623-1629.
- 23. Newhook LA, Curtis J, Hagerty D, et al. High incidence of childhood type 1 diabetes in the Avalon Peninsula, Newfoundland, Canada. Diabetes Care 2004; 27: 885-888.
- 24. Gale EA, Gillespie KM. Diabetes and gender. Diabetologia 2001; 44: 3-15.
- 25. Lonnrot M, Knip M, Roivainen M, et al. Onset of type 1 diabetes mellitus in infancy after enterovirus infections. Diabet Med 1998; 15: 431-434.
- 26. Lonnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort. The Finnish Diabetes Prediction and Prevention Study. Diabetes 2000; 49: 1314-1318.
- 27. Salminen K, Sadeharju K, Lonnrot M, et al. Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol 2003; 69: 91-98.
- 28. Viskari HR, Koskela P, Lonnrot M, et al. Can enterovirus infections explain the increasing incidence of type 1 diabetes? Diabetes Care 2000; 23: 414-416.
- 29. Libman IM, Pietropaolo M, Arslanian SA, et al. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care 2003; 26: 2871-2875.





Abstract
Objectives: To determine the incidence of childhood type 1 diabetes mellitus (T1DM) in New South Wales from 1997 to 2002; to compare with previously published rates (1990–1996); and to analyse trends in incidence from 1990 to 2002.
Design, setting and participants: Prospective population-based incidence study. Primary ascertainment of incident cases aged < 15 years was from the Australasian Paediatric Endocrine Group NSW children’s diabetes register. Secondary ascertainment was from the National Diabetes Supply Scheme until 1999 and from the Australian Institute of Health and Welfare thereafter. Childhood population data were obtained from the Australian Bureau of Statistics.
Main outcome measures: Age-standardised incidence; trends in incidence by calendar year, and sex and age at diagnosis.
Results: There were 3260 incident cases (1629 boys, 1631 girls) in the 13 years. Case ascertainment was 99.7% complete using the capture–recapture method. Mean age-standardised incidence per 100 000 person-years was 20.9 (95% CI, 19.9 to 21.9) from 1997 to 2002 compared with 17.8 (95% CI, 17.0 to 18.7) from 1990 to 1996; there was a plateau in incidence between 1997 and 2002. Overall, the incidence increased on average by 2.8% per year (95% CI, 1.9% to 3.8%, P < 0.001) and increased with age, being 12.2 (95% CI, 11.3 to 13.1) in 0–4 year olds; 18.9 (95% CI, 17.8 to 20.0) in 5–9 year olds and 26.7 (95% CI, 25.4 to 28.1) in 10–14 year olds. The increase per year in 0–4 year olds (3.9%) was not significantly higher than in older children. The mean incidence of T1DM was 19.8 (95% CI, 18.8 to 20.7) in girls and 18.8 (95% CI, 17.9 to 19.7) in boys (P = 0.02).
Conclusions: The incidence of childhood-onset T1DM has increased significantly in all age groups in NSW since 1990. Resource planning in the management of childhood diabetes in NSW should take these findings into account.