Community-acquired pneumonia (CAP) causes significant morbidity, with an overall mortality of 6%–8% in patients admitted to hospital.1 The hospital-in-the-home care model is safe and effective.2-4 However, the popularity of hospital in the home for CAP varies, as some doctors consider patients requiring intravenous (IV) antibiotics too unwell for home treatment.5 A recent study demonstrated that outpatient treatment with oral antibiotics is safe and effective for mild CAP.6 Although CAP patients have been included in small numbers in some general trials of hospital in the home, there are no randomised controlled trials of hospital in the home versus hospital care of patients with mild to moderate CAP.
Pegasus Health is an Independent Practitioners Association of about 230 (70%) general practitioners in Christchurch, New Zealand. The organisation set up an “Extended Care @ Home” service, providing extended medical and nursing care to patients in their homes. Our hypothesis was that patients with mild to moderate pneumonia could be effectively treated at home by primary care teams using this model.
We carried out a randomised controlled trial of home versus hospital (usual) care of mild to moderate CAP. This study was approved by the Canterbury Ethics Committee, Christchurch, NZ.
All patients attending the emergency department (ED) at Christchurch Hospital from July 2002 to October 2003 with a clinical diagnosis of CAP who met the eligibility criteria were invited to participate. CAP was defined according to the British Thoracic Society criteria:
An acute respiratory tract illness associated with radiographic shadowing consistent with infection on a chest radiograph (CXR) which was neither pre-existing nor of any other known cause.7
A formal severity assessment was performed using the CURB-65 score.7 This simple validated scoring system is practical to implement in a busy ED and enables stratification of patients with CAP into mortality risk groups which might be suitable for different management options.1,7 Items contributing to the score are confusion, blood urea concentration, respiratory rate, blood pressure and age. Patients with a CURB-65 score of 0–2 have a low mortality (0.7%–9.2%) and were considered for management in the community.7
The following additional exclusion criteria were applied: living outside the Christchurch metropolitan area; in hospital-level accommodation or of no fixed abode; living alone with no alternative accommodation; serious comorbidity requiring hospital treatment;7 pneumonia which was not the primary cause for hospital admission, was distal to bronchial obstruction or was associated with pleural effusion; and expected death. Patients who had tuberculosis, bronchiectasis or HIV infection or were immunocompromised were also excluded, along with patients who had been in hospital within the previous 14 days, had pulse oximetry oxygen saturation < 92% on air, or had previously been entered in the study.
Patient characteristics, patient-rated symptom severity and general functioning using the SF-12 scale8 were recorded by the study team at trial entry (the SF-12 yields two summary measures of functional physical and mental health).
Microbiological samples were obtained, and included sputum where available for Gram stain, culture and sensitivity (including Legionella pneumophila), urine for Streptococcus pneumoniae and L. pneumoniae antigen testing, standard serological testing for L. pneumoniae and Mycoplasma pneumoniae antibodies, blood cultures and a nasopharyngeal swab for Bordetella pertussis culture.
EC@H is provided by a GP Medical Director and experienced primary care nurses in conjunction with the patient’s own primary care team. It covers a similar range of activities to hospital in the home, providing an IV antibiotic service using standard cannulae, home support services, short-term home nursing care and mobile diagnostic testing.2,9,10
All patients in both groups received an initial dose of IV antibiotics while in the ED. Identical antibiotic treatment guidelines were provided, but choice was left to the physicians in both groups. The protocol provided guidelines for recognising failure of treatment or development of complications requiring consideration of secondary care transfer.
Initially, patients randomised to home care had a daily visit from a GP and at least twice-daily visits by a nurse. Initial chest x-rays of community patients were reviewed by a respiratory physician, and any requiring follow-up were highlighted.
Home care patients were given a 24-hour emergency contact number and a list of symptoms that should prompt contact.
The primary outcome measures were duration to discharge from hospital or EC@H program, duration of IV and subsequent oral antibiotics, self-rated symptom severity, and general functioning using the SF-12 scale. Secondary outcome measures were complications and patient satisfaction. Patient-perceived symptom severity was recorded using a previously developed pneumonia symptom score.11 There were additional questions on sleep and appetite disturbance. Self-rated symptom severity, general functioning and adverse events were recorded daily. Data on duration of admission and antibiotics were extracted from the case records.
Patients were contacted by telephone at 2 and at 6 weeks after presentation to record satisfaction, self-rated symptom severity and functional outcome data using the SF-12 scale. Costs were calculated from a funder’s perspective. Direct costs of providing home care were extracted for each patient to align as closely as possible with the hospital costing formula, for which the Victorian diagnosis-related groups (DRGs) system version 4.2 is used,12 and included actual costs for each study patient for staff time and transport, equipment, pharmaceuticals, and support services such as home help, as well as mean administrative, laboratory and radiology costs per patient for the EC@H service.
The study ran from July 2002 until October 2003. Fifty-five patients met the initial inclusion criteria, consented and were randomised. There were six exclusions after randomisation (Box 1). Complete data for primary and secondary outcome measures were obtained from all remaining patients. The demographic and clinical characteristics of the two patient groups were similar (Box 2). A screening log was kept, covering representative time periods at the beginning, middle and end of the study, which totalled 8 months of the 15-month study period. During this time, 288 patients with potential CAP were identified in ED triage codes and from chest x-ray results. Of these, 208 did not fit the inclusion criteria. These estimates were extrapolated to estimate the eligible population for the entire period (Box 1).
A specific bacterial diagnosis was made in nine out of 24 patients in the home arm and in 13 out of 25 patients enrolled in the hospital arm. M. pneumoniae diagnosed serologically was the most common pathogen identified in the patients treated at home (five out of 24 patients), followed by S. pneumoniae (in two patients). In the hospital arm, S. pneumoniae was the most frequently isolated pathogen (3/25 detected with the urinary antigen detection test and 3/25 isolated in sputum culture), followed by M. pneumoniae (3/25) and L. pneumophila (2/25), both diagnosed serologically.
Our results suggest that home treatment of mild to moderately severe pneumonia, assessed using the CURB-65 scoring system, is an effective alternative to hospital treatment.
Although the duration of IV and oral antibiotics was similar, the time to discharge was significantly longer for patients cared for in the home. This was probably accounted for by the EC@H guideline, which requires patients to have been afebrile for two consecutive readings and observed by EC@H for 48 hours before change to oral antibiotics. In contrast, some patients treated in hospital were discharged before 48 hours. There were no clinically significant differences in median time to clinical sign resolution or in resolution of patient-reported symptoms. General functioning assessed by the SF-12 was similar in both groups, and complications had a similar frequency and pattern. Patients were appropriately referred back to hospital and there were no serious adverse events. The study was not powered to show differences in mortality. Very large numbers would be required for such a study, as patients at low risk of mortality were selected.
This model of care is very acceptable to patients, with a high level of satisfaction with care in the home environment. These are additional outcomes that show advantage, and therefore have the potential to be type I errors, but at the very least they do not show inferiority of the home program.
Caseweight-based costs are a blunt tool for cost comparison. Actual costs of hospital care are not available. However, using this tool, the costs of location of treatment to the funder can be compared. Using this model, the EC@H model of care appears cost effective, with care of the home patients costing the funder around three-quarters of the caseweight-based cost of care for hospital care of these patients.
Some patients potentially eligible for the study were not offered enrolment. This may have been for logistic reasons in the ED or a later diagnosis.
The strength of this study is that it tests a unique model of care for CAP that to our knowledge has never been prospectively evaluated in a randomised controlled trial. This model has previously been tested for cellulitis.13
Our results build on recent evidence that outpatient treatment of mild pneumonia is safe.6 A proportion of patients with the mildest pneumonia in the CURB 0–2 range can be safely treated with oral antibiotics and are likely to require less intense review. Most mild pneumonia is probably already treated in the community with oral antibiotics, and more than half of this group had already had a trial of oral antibiotics before referral. It is important not to treat patients in this group with IV antibiotics if evidence indicates they are suitable for oral antibiotics.
Equally, for people with more severe pneumonia, the careful selection and close monitoring described in this study are very important, and our findings cannot necessarily be extrapolated to less intensive models.
Community-based care of serious conditions such as pneumonia has traditionally been organised by secondary care programs. These have generally involved admission under a medical team with early discharge to the community. EC@H is the first model where the full clinical responsibility for patients with CAP is taken by primary care teams. This not only allows patients to remain at home, but also increases the skills and experience of the GP workforce in managing acutely unwell patients in the home environment.
1 Participant flow diagram
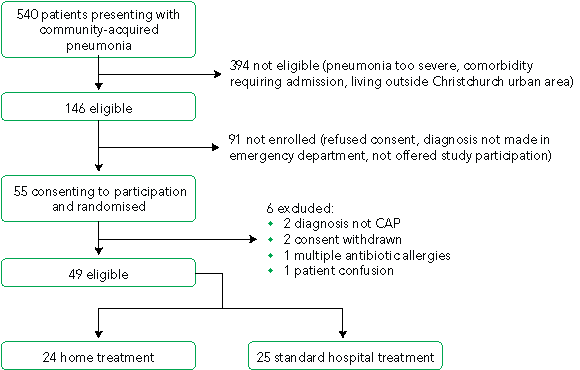
All patients in both groups completed questionnaires at baseline, at 2 weeks, and at 6 weeks. The numbers of eligible and ineligible patients were estimated from the screening log (which covered 8 of the 15 months).
2 Patient characteristics
|
Home (n = 24) |
Hospital (n = 25) |
|||||||||||||
Sex |
|
|
|||||||||||||
Male |
13 |
13 |
|||||||||||||
Female |
11 |
12 |
|||||||||||||
Ethnicity |
|
|
|||||||||||||
New Zealand European |
20 |
13 |
|||||||||||||
Other European |
2 |
7 |
|||||||||||||
Maori |
0 |
3 |
|||||||||||||
Pacific Island |
2 |
1 |
|||||||||||||
Other |
0 |
1 |
|||||||||||||
Mean age (years) |
50.1 |
49.8 |
|||||||||||||
High user health card* |
3 |
2 |
|||||||||||||
Community services card† |
9 |
9 |
|||||||||||||
Highest education level‡ |
|
|
|||||||||||||
Primary |
1 |
1 |
|||||||||||||
Secondary |
10 |
18 |
|||||||||||||
Tertiary |
13 |
6 |
|||||||||||||
Smoking status |
|
|
|||||||||||||
Never |
10 |
10 |
|||||||||||||
Current smoker |
4 |
7 |
|||||||||||||
Ex-smoker |
10 |
8 |
|||||||||||||
Mean pack-years |
18.9 |
20.4 |
|||||||||||||
Prior antibiotics |
14 |
10 |
|||||||||||||
Other medical conditions (mean number) |
0.8 |
1.1 |
|||||||||||||
CURB-65 score |
|
|
|||||||||||||
0 |
13 |
14 |
|||||||||||||
1 |
6 |
8 |
|||||||||||||
2 |
5 |
3 |
|||||||||||||
* Card providing access to sudsidised health care and medicines for frequent users of the health system. |
|||||||||||||||
3 Mean physical and mental component scores of SF-12*
|
Home |
Hospital |
P |
||||||||||||
Physical component |
|||||||||||||||
2 weeks |
38.1 |
40.2 |
0.45 |
||||||||||||
6 weeks |
42.2 |
45.8 |
0.18 |
||||||||||||
Mental component |
|||||||||||||||
2 weeks |
48.3 |
48.6 |
0.91 |
||||||||||||
6 weeks |
50.4 |
51.0 |
0.81 |
||||||||||||
*A higher score represents better functioning. |
|||||||||||||||
Received 31 March 2005, accepted 30 June 2005
- Dee A Richards1
- Les J Toop2
- Michael J Epton3
- G Ian Town4
- Robin D Dawson5
- Michael C Hlavac6
- Graham R B McGeoch7
- Simon M H Wynn-Thomas8
- Paul D Abernethy9
- Anja M Werno10
- 1 Christchurch School of Medicine and Health Sciences, Otago University, Christchurch, NZ.
- 2 Pegasus Health Independent Practitioners Association, Christchurch, NZ.
- 3 Department of Microbiology, Canterbury Health Laboratories, Christchurch, NZ.
We thank the following for their help with the study: the Pegasus Health Extended Care team, especially Chris Tallott; members of the Christchurch CAP Research Team: Professor David Murdoch (Microbiology), Professor Stephen Chambers (Infectious Diseases), Dr Lance Jennings (Virology), Dr Richard Laing and Dr Martin Kelly (Respiratory Medicine), Dr Alan Pithie (General Medicine), Dr Martin Than and Dr Jan Bone (Emergency Medicine), Associate Professor Chris Frampton (biostatistician); the Emergency Department and General Medical teams; the staff of the Microbiology Department, especially Kirsten Beynon, Margaret Sutherland, Felicity Beats and Toni Stewart (Research Nurses) and Alison Parsons (Research Assistant).
None identified.
- 1. Neill A, Martin I, Weir R, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax 1996; 51: 1010-1016.
- 2. Caplan GA, Ward JA, Brennan NJ, et al. Hospital in the home: a randomised controlled trial. Med J Aust 1999; 170: 156-160. <MJA full text>
- 3. Grayson ML. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Aust 1995; 162: 249-253.
- 4. Montalto M. Patients’ and carers’ satisfaction with hospital-in-the-home care. Int J Qual Health Care 1996; 8: 243-251.
- 5. Howden BP, Grayson ML. Hospital-in-the-home treatment of infectious diseases. Med J Aust 2002; 176: 440-445. <MJA full text>
- 6. Carratala J, Fernandez-Sabe N, Ortega L, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med 2005; 142: 165-172.
- 7. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377-382.
- 8. Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997; 19: 179-186.
- 9. Board N, Breenan NJ, Caplan GA. A randomised controlled trial of the costs of hospital as compared with hospital in the home for acute medical patients. Aust N Z J Public Health 2000; 24: 305-311.
- 10. Grayson ML. Hospital in the home development project. Development of Victorian disease-specific guidelines for hospital in the home (HITH). Final report. Melbourne: Monash Medical Centre, 1999.
- 11. Metlay JP, Fine MJ, Schultz R, et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med 1997; 12: 423-430.
- 12. Folland S, Goodman AC, Stano M. Government regulation — principal regulatory mechanisms. In: The economics of health and health care. 3rd ed. New Jersey: Prentice Hall, 2001.
- 13. Corwin P, Toop L, McGeoch G, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005; 330: 129-132.





Abstract
Objective: To determine whether community management of mild to moderate community-acquired pneumonia (CAP) is as effective and acceptable as standard hospital management of CAP.
Design: Randomised controlled trial.
Setting: Christchurch, New Zealand, primary and secondary care.
Participants: 55 patients presenting or referred to the emergency department at Christchurch Hospital with mild to moderately severe pneumonia, assessed using a validated pneumonia severity assessment score, from July 2002 to October 2003.
Interventions: Hospital treatment as usual or comprehensive care in the home delivered by primary care teams.
Main outcome measures: Primary: days to discharge, days on intravenous (IV) antibiotics, patient-rated symptom scores. Secondary: health status measured using level of functioning at 2 and 6 weeks, patient satisfaction.
Results: The median number of days to discharge was higher in the home care group (4 days; range, 1–14) than in the hospital groups (2 days; range, 0–10; P = 0.004). There was no difference in the number of days on IV antibiotics or on subsequent oral antibiotics. Patient-rated symptom scores at 2 and 6 weeks, median change in symptom severity from baseline to 6 weeks, and general functioning at 2 and 6 weeks did not differ between the groups. Patients in both groups were satisfied with their treatment, with a clear preference for community treatment (P < 0.001).
Conclusions: Mild to moderately severe CAP can be managed effectively in the community by primary care teams. This model of comprehensive care at home can be implemented by primary care teams with suitable funding structures.