Smoking rates in Australia continued to fall in the past decade, from 27% of Australians in 1989 to 23% in 2001. This consolidated and extended the previous decade’s more dramatic reduction in smoking prevalence, from 35% in 1980.1 Despite this progress, smoking is still the leading risk factor for total disease burden in Australia, causing almost 10% of disability-adjusted life-years (DALYs) lost.2 Further reductions in smoking are achievable. For example, smoking prevalence had dropped to 16.4% in California and 19% in Massachusetts by 2002.3 Sustained mass-media-led tobacco control campaigns conducted in these states through the 1990s have been credited with producing these smoking rate reductions in a cost-effective manner.4
Information on the health and economic benefits of further reductions in smoking rates has the potential to persuade Australian health policy-makers to increase their investment in tobacco-control programs. The purpose of this study was therefore to estimate the short-term benefits of a reduction in smoking on acute myocardial infarction (AMI) and stroke hospitalisations. The specific goal was to estimate the numbers of AMI and stroke hospitalisations in 35–64 year-olds, and the associated health care costs, that could have been avoided over a 7-year period in Australia from the 2001–02 financial year, under two smoking prevalence reduction scenarios.
Scenario 1 was a 1% absolute reduction in 2001–02 (Year 1), and Scenario 2 was a 5% absolute reduction, achieved through 1% per annum reductions in five consecutive years from 2001–2002. AMIs and stroke were chosen because together they are the leading cause of total disease burden in Australia, accounting for nearly 18% of DALYs lost.2 Ischaemic heart disease is the leading cause of admission to hospital associated with smoking, and 72% of admissions attributable to tobacco are for 35–64-year-olds.5 The two scenarios are potentially achievable. For example, smoking prevalence dropped 1.7% in the initial 7-month phase of the 1997 Australian National Tobacco Campaign, and falls comparable to Scenario 2 were seen in Massachusetts in the 1990s during its sustained tobacco-control campaign.4
A similar study was conducted by Lightwood and Glantz for the US population in 1990.6 Their model was applied to the English population in 2000.7 We updated and extended the method and applied it to the Australian population. There were three steps:
Estimation of the fall over time in risk of AMI and stroke after smoking cessation;
Calculation of the mean hospitalisation rates for never-smokers and ex-smokers (by year since quitting), and the mean costs of AMI and stroke; and
Simulations to estimate the distributions of the numbers of AMI and stroke hospitalisations avoided and the cost savings.
Lightwood and Glantz estimated functions for the relative risk (RR) of AMI and stroke for ex-smokers, from the time since quitting.6 Data on RRs came from studies that met pre-specified criteria. They had to be case–control or population-based studies, reporting RRs adjusted for age and pre-existing cardiovascular and cerebrovascular diseases (if patients with these diseases had not been excluded from analysis). Studies on heart attacks had to report the RR for AMI specifically. At the time, five studies met the criteria for AMI,8-12 and two for stroke.13,14
A literature search for more recent studies identified one study on AMI,15 but none on stroke. The new AMI study (McElduff et al15) was used instead of the AMI study by Dobson et al,12 which had been included in the Lightwood and Glantz analysis, as it updated the Dobson et al analysis and included over four times as many cases.
Functions were re-estimated for both stroke (males and females combined) and AMI (males and females separately) to source the standard errors for the subsequent simulations. The non-linear platform in the statistics package, JMP version 5.1, 2003 (SAS Institute, Cary, NC, USA), was used to fit appropriate functions to the relative risk–time coordinates from the articles. The logarithms of the relative risks were weighted according to their standard errors, which were calculated from their confidence intervals.
Non-linear models were fitted of the risks of AMI and stroke for ex-smokers relative to never-smokers as a function of time (t months) since quitting. For stroke the function had three parameters:
RR0 = the RR of current smokers versus never-smokers;
RR∞ = the RR of long-term ex-smokers versus never-smokers; and
τ = a slope parameter that determines how quickly the RR decreases with time since quitting.
As sufficient data were available for AMI to differentiate by sex, additional parameters RR0F and RR∞F were included in the model, where F is an indicator variable for female.
The model for stroke was:
lnRR(t) = ln{[RR0 − RR∞]e−t/τ + RR∞} + ε
The model for AMI was:
lnRR(t) = ln{[(RR0 + RR0FF) − (RR∞ + RR∞F)]e−t/τ + (RR∞ + RR∞F)} + ε
The model parameters and standard errors are presented in Box 1, and the relative risk functions for AMI and stroke are graphed in Box 2. These functions show that the excess risk of AMI and stroke associated with smoking declines rapidly after quitting, and that this decline is faster for AMI than stroke. For long-time ex-smokers, the risk of AMI reverts almost to that of non-smokers, especially for men (RR∞ = 1.101 for men and RR∞ + RR∞F = 1.319 for women). The risk of stroke remains slightly elevated (RR∞ = 1.438). The differences between the model parameters and those derived by Lightwood and Glantz were slight.
The number of hospitalisations avoided [hA(s)] in year s for people who quit smoking t years ago is:
hA(s) = qNcsr(0) − Nqsr(t)
where q is the proportion of quitters in the cohort, Ncs is the number of current smokers remaining in the cohort in year s, Nqs is the number of quitters remaining in in year s, and r(t) is the rate of hospitalisation for an individual who quit t years ago.
The above incidences and numbers of hospitalisations avoided were calculated using the Australian data in Box 3. The proportions of smokers and ex-smokers were obtained from the 2001 National Drug Strategy Household Survey. Counts of hospitalisations (separations) for AMI and stroke in 2001–02 were obtained from the Australian Institute of Health and Welfare (AIHW) and had been sourced from the National Hospital Morbidity Database (http://www.aihw.gov.au/hospitaldata/morbidity.html [no longer accessible]). Population data for 2001 from the Australian Bureau of Statistics were used to calculate the hospitalisation incidences, r0. The annual survival probabilities (in smokers who had never had an event) were estimated from the 1995–1997 Australian life tables. Annual survival probabilities after an AMI (unpublished Health Department data supplied by Associate Professor Michael Hobbs, School of Population Health, University of Western Australia), and stroke16 were estimated from Western Australian data.
Cost savings were the number of hospitalisations avoided multiplied by the mean cost associated with hospitalisation and subsequent health care.
The average costs of AMI for the 1st year were estimated from AIHW data on the costs of admissions and revascularisations and the proportion of patients having revascularisation procedures during acute admissions (sourced from the National Hospital Morbidity Database and the National Hospital Cost Data Collection), and Western Australian data on the proportion of patients having revascularisations within a year.17 Costs in the 2nd and 3rd years after an AMI were assumed to be the same proportion of 1st year costs as reported by Lightwood and Glantz.6
The average costs of stroke were estimated from an Australian study by Dewey et al.18 First year direct costs for a first-ever stroke were adjusted to 2001 prices using the health service index from the Consumer Price Index. Eighty-seven per cent of these costs were for hospitalisation or nursing-home care. The average costs for 2nd and subsequent years were estimated from the lifetime cost estimate of Dewey et al,18 adjusting for indirect costs, 1st-year costs, survival and prices.
The numbers of hospitalisations and strokes avoided were estimated for the cohort of 35–64-year-olds by simulation, using the equations above and the program JMP 5.1 (SAS Institute).
The simulation randomly generated 10 000 observations for each of the random variables. It then used these random observations to calculate the number of cases of AMI and stroke for each year for 7 years for the cohort of quitters and the number of cases if the cohort had not quit smoking, for both smoking prevalence-reduction scenarios. The proportions of smokers and ex-smokers to have AMI or stroke events and the corresponding mortality rates were treated as normally distributed random variables, to incorporate the uncertainty of these estimates into the simulation. The AMI and stroke risk reductions as a function of number of years since quitting smoking were also simulated randomly to incorporate the uncertainty in the parameter estimates used in this function. The other parameters, such as actual number of quitters, and costs per event, were entered as constants.
Cumulative numbers of hospitalisations avoided and costs were calculated for each year in the 7-year period. Cumulative cases and costs were discounted to present value (Year 1) at a rate of 5% per annum. The Lightwood and Glantz methodology was also extended to track the number of cases and expenditures for the population of 35–64-year-olds. Population growth or smoking-rate trends were not incorporated. This simplification would not affect the outcomes, if it is assumed that the number of quitters is proportional to any such trends.
For validation purposes, a simulation was first run with the US data used by Lightwood and Glantz. A very similar set of results to that reported in Table 3 of Lightwood and Glantz was obtained, with minor differences due to the random error expected in simulations, and the updated RR function.6
Hospitalisations avoided and cost savings in Australia for Scenarios 1 and 2 are shown in Box 4. The results predict that a 1% drop in smoking prevalence would avoid almost 1000 hospitalisations for AMI, and about 350 hospitalisations for stroke over the ensuing 7 years. About $20.4 million in health care costs could be saved — almost 1% of the costs for treating AMI and stroke in 35–64-year-olds over that period.
Scenario 2 produces substantially greater benefits. A 5% reduction in smoking prevalence over 5 years would avoid over 3000 AMI hospitalisations and over 1000 stroke hospitalisations over a 7-year period, and could save $61.6 million in health care costs — 2.75% of costs for AMI and stroke over the period.
This study provides further support for the proposition that modest and achievable reductions in smoking rates can substantially improve health outcomes and reduce health care costs. Furthermore, this study demonstrates substantial health and economic benefits in the short term (7 years), in contrast to previous projections of economic benefits from reduced use of pharmaceuticals, which spanned the 40-year period of the Commonwealth Government’s Intergenerational Report.19
This study used standard epidemiological methods which had previously been applied to the United States6 and English7 populations. The RR functions and hospitalisation-rate data, which underpinned the results, came from large data sets. For AMI, the former was estimated from three case–control studies that included over 8000 cases and over 11 000 controls,8,9,15 and two cohort studies that included almost 700 cases and over 200 000 subjects.10,11 For stroke, the RR function was derived from two cohort studies that included over 600 cases and over 120 000 subjects.13,14 Hospitalisation rates were computed from a census of separations, rather than a sample.
The benefits of smoking cessation were clearly underestimated in this study, as the only health outcomes considered were hospitalisation for AMI or stroke. I did not consider reductions in AMI and stroke mortality, or improvements in health outcomes and associated reductions in health care costs that would result from reduced incidence of the many other smoking-related diseases, such as lung cancer and chronic obstructive pulmonary disease. In fact, this study also underestimated the benefits of reduced smoking on AMI and stroke hospitalisation rates and costs, for three reasons. First, only admissions in 35–64-year-olds were included, as most (72%) ischaemic heart disease admissions attributable to tobacco are in this age group. Second, the cost estimates, particularly for AMI, did not include all health care costs subsequent to a hospitalisation. For example, the cost of pharmaceuticals (such as lipid-lowering therapy) post-AMI was not considered. Finally, passive smoking was not considered. Two studies recently published in the British Medical Journal support an association between passive smoking and coronary heart disease.20,21 In the first, hospital admissions for AMI in a geographically isolated community in the United States decreased significantly during a 6-month period in 2002 when smoking was banned in public and workplaces.20 In the second study, of over 2000 British men who were non-smokers, the incidence of coronary heart disease was about 50% higher in men with cotinine levels in the second, third and fourth quartiles than in men with lower cotinine levels.21 Cotinine is a biomarker of nicotine and, in non-smokers, provides a quantitative measure of passive smoking.
The study did have some limitations. AMI and stroke survival data were available only for Western Australia,17 and, although the validity of extrapolating these data to Australian patients cannot be confirmed, there is no reason to believe that outcomes would differ substantially between states. AMI hospitalisation costs were estimated from Australian and Western Australian data, and stroke hospitalisation costs were sourced from a Melbourne study. Although, again, the validity of extrapolating state-specific data cannot be confirmed, there was < 5% variation between states in the AMI cost data sourced from the National Hospital Cost Data Collection.
Over the past 10-year period, the Commonwealth Government has committed an average of only $2.033 million per year to public health programs aimed at tobacco harm minimisation.4 During a 7-month period when expenditure on the National Tobacco Campaign exceeded this average, smoking rates fell dramatically. Commonwealth and state expenditures on this campaign were about $9 million, and smoking prevalence dropped by 1.7%.4 This analysis found that if smoking prevalence had fallen by only 1%, these expenditures would have been more than offset in less than 4 years by savings in hospitalisations for AMI and stroke alone. Even if further similar reductions in smoking prevalence can only be achieved with more intensive (and costly) campaigns, the cost-effectiveness profile of such mass media campaigns would be likely to be favourable. For example, if it cost $13 million for a campaign that reduced smoking prevalence by 1%, the cost would be recovered through reduced hospitalisations for AMI and stroke alone in 5 years (Box 4). Advances in controlling the epidemic of coronary heart disease were made in Australia in the 1990s, with falling incidence and mortality.17 Even modest further reductions in smoking rates will make a major contribution to further control of this epidemic.
1 Fall in risk of acute myocardial infarction (AMI) and stroke over time after smoking cessation
Parameter |
Estimate |
Standard error |
|||||||||||||
AMI |
|
|
|||||||||||||
RR0 |
3.388 |
0.2147 |
|||||||||||||
RR∞ |
1.101 |
0.0801 |
|||||||||||||
RR0F |
0.637 |
0.3060 |
|||||||||||||
RR∞F |
0.218 |
0.1683 |
|||||||||||||
τ, months |
17.078 |
3.9460 |
|||||||||||||
Stroke |
|
|
|||||||||||||
RR0 |
2.804 |
0.1712 |
|||||||||||||
RR∞ |
1.438 |
0.1850 |
|||||||||||||
τ, months |
16.705 |
12.6257 |
|||||||||||||
RR0 = risk for current smokers v never-smokers. RR∞ = risk for long-term ex-smokers v never-smokers. (Note that when RR = 1, the risk for ex-smokers is the same as the risk for never-smokers.) RR0F = indicator variable to calculate the RR for females at time 0. RR∞F = indicator variable to calculate the RR for females who are long-term ex-smokers. (Note that RRs for females are RR0 + RR0F and RR∞ + RR∞F). τ, months = a slope parameter that represents the number of months it takes for the RR to decrease by a log factor. |
|||||||||||||||
2 Estimated decline in relative risk (RR) after quitting smoking
Acute myocardial infarction
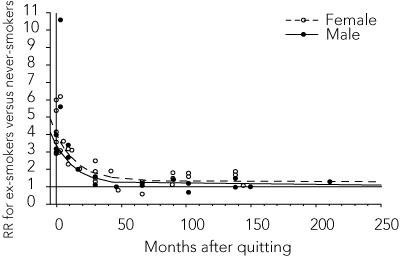
Stroke
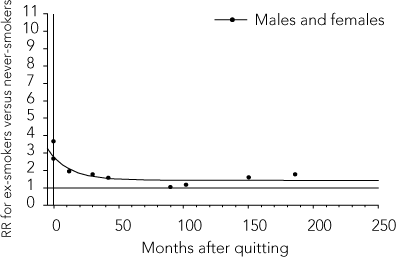
3 Parameters for simulation, Australia, 2001–2002
|
Acute myocardial infarction |
Stroke |
|
||||||||||||
|
Male |
Female |
|
||||||||||||
Proportion of smokers, pS (SE)* |
0.2456 (0.00553) |
0.2083 (0.00522) |
0.2270 (0.00381) |
|
|||||||||||
Proportion of ex-smokers, px (SE)* |
0.3597 (0.00617) |
0.2838 (0.0058) |
0.3219 (0.00425) |
|
|||||||||||
Observed hospitalisation incidence in the population, r0 |
0.0033979 |
0.0008552 |
0.0012191 |
|
|||||||||||
35–64-year-old cohort size, N |
3 570 449 |
3 625 913 |
7 196 362 |
|
|||||||||||
No. of smokers equivalent to 1% drop in prevalence† |
35 704 |
36 259 |
71 964 |
|
|||||||||||
Annual survival probability |
0.99561 |
0.99749 |
0.99656 |
|
|||||||||||
Annual survival probability after event |
|
|
|
|
|||||||||||
1st year |
0.9676 |
0.9350 |
0.6400 |
|
|||||||||||
2nd and following years |
0.9903 |
0.98147 |
0.8900 |
|
|||||||||||
Average cost of event |
|
|
|
|
|||||||||||
1st year |
$9 657 |
$8 694 |
$18 259 |
||||||||||||
2nd year |
$600 |
$513 |
$4 558 |
||||||||||||
3rd year |
$396 |
$328 |
$4 558 |
||||||||||||
4th year |
|
|
$4 558 |
||||||||||||
5th year |
|
|
$4 558 |
||||||||||||
6th year |
|
|
$4 558 |
||||||||||||
* In 35–64-year-olds. †A 1% absolute reduction (ie, from a prevalence of 0.227 in males and females combined to 0.217). |
|||||||||||||||
4 Predicted health outcomes and cost savings associated with Scenario 1 and Scenario 2
|
Scenario 1 (one time 1% drop in smoking prevalence in Year 1) |
Scenario 2 (annual 1% drop in smoking prevalence in Years 1 to 5) |
|||||||||||||
|
Year 1 |
Year 5 |
Year 7 |
Year 1 |
Year 5 |
Year 7 |
|||||||||
Cumulative hospitalisations avoided (% of population) |
|
|
|||||||||||||
AMI |
64 (0.42%) |
690 (1.01%) |
999 (1.10%) |
64 (0.42%) |
1737 (2.54%) |
3181 (3.50%) |
|||||||||
Stroke |
23 (0.26%) |
245 (0.62%) |
357 (0.68%) |
23 (0.26%) |
615 (1.56%) |
1132 (2.15%) |
|||||||||
Cumulative cost savings in $000s (SD) |
|
|
|||||||||||||
AMI |
604 (200) |
6 924 (658) |
10 141 (829) |
604 (200) |
17 218 (2180) |
31 981 (3240) |
|||||||||
Stroke |
413 (306) |
6 164 (1343) |
10 299 (1871) |
413 (306) |
14 245 (4003) |
29 646 (6691) |
|||||||||
AMI and stroke |
1 016 (366) |
13 088 (1749) |
20 440 (2046) |
1016 (366) |
31 463 (5795) |
61 626 (7434) |
|||||||||
Percent of population costs saved |
0.33% |
0.81% |
0.91% |
0.33% |
1.95% |
2.75% |
|||||||||
AMI = acute myocardial infarction. |
|||||||||||||||
Bainbridge Consultants, Melbourne, VIC.
Susan F Hurley, MPharm, MS(Biostatistics), PhD, Health Economist; and Associate Professor, School of Population Health, University of Melbourne.
©The Medical Journal of Australia 2005 www.mja.com.au PRINT ISSN: 0025-729X ONLINE ISSN: 1326-5377
Received 16 November 2004, accepted 9 May 2005
- Susan F Hurley1,2
- 1 Bainbridge Consultants, Melbourne, VIC.
- 2 School of Population Health, University of Melbourne, Melbourne, VIC.
This research was supported with funding from the Victorian Health Promotion Foundation and The Cancer Council, Victoria. These funding bodies per se had no role in the design, conduct or interpretation of the study. Susan Hurley is a consultant to the Cancer Council, Victoria. Dr Gunter Hartel assisted with simulations and analyses. Xia (Cherie) Du provided hospitalisation counts, and Associate Professor Michael Hobbs provided AMI survival data.
None identified.
- 1. White V, Hill D, Siahpush M, Bobevski I. How has the prevalence of cigarette smoking changed among Australian adults? Trends in smoking prevalence between 1980 and 2001. Tobacco Control 2003; 12 Suppl II: ii67-ii74.
- 2. Mathers CD, Vos ET, Stevenson CE, Begg SJ. The Australian Burden of Disease Study: measuring the loss of health from diseases, injuries and risk factors. Med J Aust 2000; 172: 592-596. <MJA full text>
- 3. State-specific prevalence of current cigarette smoking among adults - United States, 2002. MMWR Morb Mortal Wkly Rep 2004; 52: 1277-1280.
- 4. VicHealth Centre for Tobacco Control. Tobacco control: a blue chip investment in public health. Melbourne: The Cancer Council, Victoria, 2003.
- 5. Ridolfo B, Stevenson C. Quantification of drug-caused mortality and morbidity in Australia, 1998. Drug statistics series, no. 7. Canberra: Australian Institute of Health and Welfare, 2001. (AIHW Cat. No. PHE-29 ed.)
- 6. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation. Circulation 1997; 96: 1089-1096.
- 7. Naidoo B, Stevens W, McPherson K. Modelling the short term consequences of smoking cessation in England on the hospitalisation rates for acute myocardial infarction and stroke. Tob Control 2004; 9: 397-400.
- 8. Rosenberg L, Kaufman DW, Helmrich SP, Shapiro S. The risk of myocardial infarction after quitting smoking in men under 55 years of age. N Engl J Med 1985; 313: 1511-1514.
- 9. Rosenberg L, Palmer JR, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. N Engl J Med 1990; 322: 213-217.
- 10. Kawachi I, Colditz G, Stampfer MJ, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Int Med 1994; 154: 169-175.
- 11. Willett WC, Hennekens CH, Bain C, et al. Cigarette smoking and non-fatal myocardial infarction in women. Am J Epidemiol 1981; 113: 575-582.
- 12. Dobson AJ, Alexander HM, Heller RF, Lloyd DM. How soon after quitting smoking does risk of heart attack decline? J Clin Epidemiol 1991; 44: 1247-1253.
- 13. Kawachi I, Colditz G, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. JAMA 1993; 269: 232-236.
- 14. Wannamethee SG, Shaper G, Whincup PH, Walker M. Smoking cessation and the risk of stroke in middle-aged men. JAMA 1995; 274: 155-160.
- 15. McElduff P, Dobson A, Beaglehole R, Jackson R. Rapid reduction in coronary risk for those who quit cigarette smoking. Aust N Z J Public Health 1998; 22: 787-791.
- 16. Hardie K, Hankey GJ, Jamrozik K, et al. Ten-year survival after first-ever stroke in the Perth community stroke study. Stroke 2003; 34: 1842-1846.
- 17. Mathur S. Epidemic of coronary heart disease and its treatment in Australia. Cardiovascular disease series, number 20. Canberra: Australian Institute of Health and Welfare, 2002.
- 18. Dewey H, Thrift AG, Mihalopoulos C, et al. Lifetime cost of stroke subtypes in Australia. Findings from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2003; 34: 2502-2507.
- 19. Hurley SF, Scollo MM, Younie SJ, et al. The potential for tobacco control to reduce PBS costs for smoking-related cardiovascular disease. Med J Aust 2004; 181: 252-255. <MJA full text>
- 20. Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ 2004; 328: 977-983.
- 21. Whincup PH, Gilg JA, Emberson JR, et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ 2004; 329: 200-205.





Abstract
Objective: To estimate the short-term benefits of a reduction in smoking on acute myocardial infarction (AMI) and stroke hospitalisations and costs.
Design and setting: Epidemiological study which applied functions describing reductions over time in risk of AMI and stroke in people quitting smoking to hospitalisation rates and costs for Australia.
Main outcome measures: The numbers of AMI and stroke hospitalisations in 35–64-year-olds and the associated costs that could have been avoided over a 7-year period from 2001–02 if smoking prevalence had decreased by 1% in the first year (Scenario 1) or by 1% per annum for 5 consecutive years (Scenario 2).
Results: Under Scenario 1, almost 1000 hospitalisations for AMI and about 350 hospitalisations for stroke would have been avoided over 7 years, saving about $20.4 million in health care costs. Under Scenario 2, over 3000 AMI hospitalisations and over 1000 stroke hospitalisations would be avoided, and health care costs could be reduced by $61.6 million (2.75% of costs for AMI and stroke over the period).
Conclusions: This study provides further support for the proposition that modest and achievable reductions in smoking rates can substantially improve health outcomes and reduce health care costs, even in the short term.