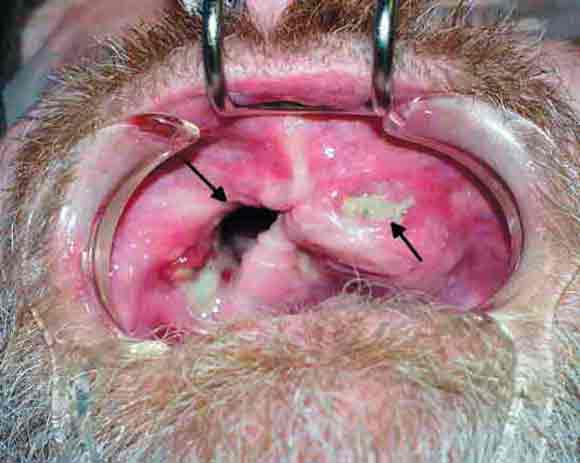
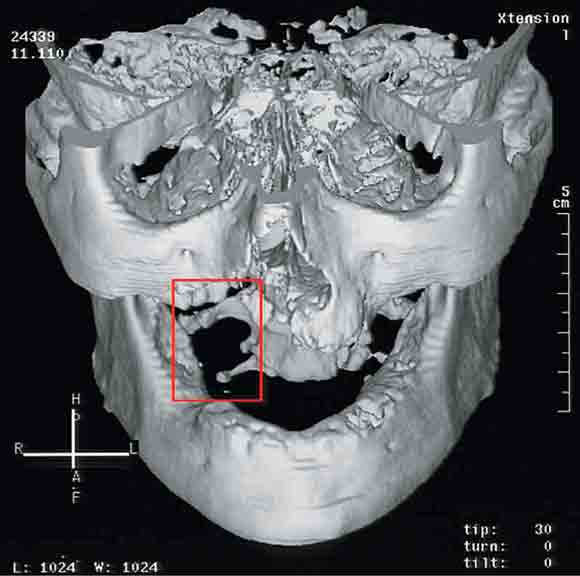
In 2003, five patients presented to the Oral and Maxillofacial Surgery Unit at Royal Adelaide Hospital, South Australia, with painful exposed bone in the maxilla, or both the maxilla and mandible (Box 1 and Box 2 ).
All patients were receiving either pamidronate (Aredia [Novartis]) or alendronate (Fosamax [Merck Sharp & Dohme]). Pamidronate was being given intravenously monthly at a dose of 60mg (one patient) or 90 mg (three patients). The patient taking alendronate received a daily oral dose of 40mg. Duration of bisphosphonate therapy was 6 months to 6 years.
Associated risk factors for the development of avascular necrosis included renal impairment in one patient and hypoproteinaemia in another.
Initial management of these patients comprised surgical debridement of the exposed bone. Histopathological assessment of surgical specimens showed no histological evidence of myelomatous deposits or Paget's disease from the affected sites in the jaws in any of the patients. None had exposed bone elsewhere in the body.
Here we present five cases of osteonecrosis of the jaw associated with bisphosphonate use. In North America, several preliminary reports have been published of unusual cases of avascular necrosis of the jaw in patients using second-and third-generation nitrogen-containing bisphosphonates.1-4 These included pamidronate, alendronate, risedronate and zoledronic acid.2,3
In Australia, bisphosphonates have been available for several years and are commonly prescribed for a range of conditions, including osteoporosis, Paget’s disease, multiple myeloma,5,6 hypercalcaemia of malignancy, and bone metastases of malignancies such as breast and prostate cancer. An estimated 200 000 patients in Australia receive these drugs. Commonly reported adverse reactions involve gastrointestinal symptoms. Bone-marrow depression and thrombocytopenia are also reported rarely.
The second- and third-generation bisphosphonates are significantly more potent than their first-generation predecessors, (etidronate, clodronate and tiludronate). They inhibit bone resorption by osteoclastic inhibition, through selective concentration at the interface of the active osteoclast and the bone-resorption surface. The specific mechanism of this inhibition is unknown, but there is evidence for several actions, including inhibition of osteoclast development from precursor cells, increase in osteoclast apoptosis,7 stimulation of osteoclast inhibitory factor, reduction of osteoclast activity, and down-regulation of matrix metalloproteinases. The resulting reduction in osteoclastic activity reduces bone resorption, supporting the use of bisphosphonates for the above indications.
However, osteoclastic function is part of the cycle of bone turnover; osteocytes have a life span of about 150 days, after which osteoclasts resorb the mineral matrix of bone and release bone morphogenetic protein and insulin-like growth factors, which in turn induce local stem cells to differentiate into osteoblasts and form new bone. This cycle is critical to maintaining bone stocks and bone viability. If osteoclastic function is too severely impaired, dead and dying osteocytes are not replaced, and the capillary network in the bone is not maintained, resulting in avascular bone necrosis.
Dental disease and denture-wearing are ubiquitous. Studies involving larger patient numbers have shown that nearly 80% of cases were initiated by tooth removal.3 Patients who have been using potent bisphosphonates for more than 6 months appear to be at highest risk.2 Other apparent risk factors are residual multiple myeloma or other malignancy, hypoproteinaemia, renal impairment from disease or drugs, and chemotherapy.
Although a definite cause-and-effect relationship is yet to be established, the association between bisphosphonate therapy and osteonecrosis of the jaw appears strong.2-4,8-10 The incidence of the potential complication appears low. In South Australia in 2003, about 14 000 patients received prescriptions for the potent second- and third-generation nitrogen-containing bisphosphonates, with about 10% having intravenous administration. Our five patients probably represented all cases of the complication, as our Department is the only oral and maxillofacial surgery service in SA and receives referrals from around the state.8,9 Checking with colleagues and related hospital services has not revealed further cases in SA, but we are aware of other cases in all states of Australia. Indeed, the Adverse Drug Reactions Advisory Committee recently reported another nine cases.10
At present, there is no effective treatment for the condition. Patients are usually referred to oral and maxillofacial surgeons, but surgical intervention is difficult as it often exposes further bone, and, as bisphosphonates affect the whole skeleton, locating viable bone margins may be impossible. Furthermore, removal of painful teeth, while initially alleviating pain, also further exposes bone, causing more pain. Covering exposed bone with tissue flaps has been found ineffective because of the development of fistulae around the flaps and possible complete dehiscence (Box 1). Compounding the problem, some patients must continue with bisphosphonate therapy and/or other chemotherapies to control hypercalcaemia. Hyperbaric oxygen therapy is not useful in bisphosphonate-induced necrosis, and antibiotics are indicated only to treat secondary infection.
Because of the lack of effective treatment for the condition, second- and third-generation bisphosphonates should be used only when benefits clearly outweigh risks. When intravenous or high-dose oral bisphosphonates are considered appropriate, referral for full dental assessment and treatment before the start of therapy should be considered. Once bisphosphonate therapy has begun, there should be regular clinical monitoring of oral health. Avoiding tooth removal and dental implants, non-surgical control of periodontal disease, and use of soft liners on dentures also seem prudent. In addition, major debridement surgeries should be avoided if at all possible.
In established cases, the primary goals are palliation and control of osteomyelitis. In most cases, progression has been controlled with long-term or intermittent courses of dicloxacillin or cephalexin (to treat any secondary infection), chlorhexidine mouthwash (Savacol), and periodic minor debridement of soft-textured sequestrating bone and wound irrigation.
The rapid expansion of indications for bisphosphonates has resulted in their widespread use across many medical disciplines, including endocrinology, rheumatology, medical oncology, haematology and general practice. Most medical practitioners are unaware of this serious and potentially permanent complication. Before prescribing bisphosphonates, medical practitioners should analyse the risks versus benefits for the individual patient, consider alternative drugs, and obtain informed consent after discussing this potential adverse reaction. We also encourage vigilant surveillance of patients who are using a bisphosphonate.
2 Clinical details of five patients with avascular necrosis of the jaw in South Australia, 2003
Age, sex |
Presentation |
Precipitant |
Bisphosphonate [indication] |
Other medications |
Treatment |
Outcome |
|||||||||
57, M |
Painful exposed bone in maxilla and mandible |
Tooth extraction |
Pamidronate (90 mg IV monthly for 6 years) [multiple myeloma] |
Dexamethasone, methotrexate, warfarin, folic acid, ranitidine, metformin, hydroxychloroquine, verapamil, sertraline, morphine |
Hyperbaric oxygen, Le Fort I level maxillectomy, bisphosphonate continued |
Persistent necrosis of midface and mandible |
|||||||||
64, M |
Ulcer in right hard palate with bone sequestrum |
Tooth extraction |
Pamidronate (90 mg IV monthly for 2 years) [multiple myeloma] |
Prednisolone, cyclosporin, itraconazole, sulfamethoxazole–trimethoprim, ranitidine, penicillin |
Sequestrectomy, local debridement, bisphosphonate continued |
Resolution |
|||||||||
73, M |
Pain, swelling of anterior maxillary alveolus |
Tooth extractions |
Alendronate (40 mg orally daily for 5 years) [Paget’s disease] |
Amlodipine, tramadol, perindopril |
Local debridement, sequestrectomies, primary flap closure, bisphosphonate ceased |
Resolution |
|||||||||
78, F |
Painful exposed bone in maxilla |
Denture pressure |
Pamidronate (90 mg IV monthly for 18 months) [Paget’s disease] |
None |
Hyperbaric oxygen, local debridement, denture reline, bisphosphonate ceased |
Persistent areas of exposed bone |
|||||||||
84, F |
Non-healing extraction site in left maxillary alveolus |
Tooth extraction |
Pamidronate (60 mg IV monthly for 6 months) [Paget’s disease] |
Diltiazem, simvastatin, ferrous sulfate, aspirin, bendrofluazide |
Wide intraoral resection with primary flap closure, bisphosphonate ceased |
Persistent fistula |
|||||||||
IV = intravenous. |
|||||||||||||||
- Glen Carter1
- Alastair N Goss2
- Chris Doecke3
- 1 Oral and Maxillofacial Surgery Unit, Royal Adelaide Hospital and Adelaide Dental Hospital, SA.
- 2 Royal Adelaide Hospital, Adelaide, SA.
- 1. Assael LA. New Foundation in understanding osteonecrosis of the jaws. J Oral Maxillofac Surg 2004; 62: 125-126.
- 2. Ruggiero S, Rosenberg TJ. Osteonecrosis of the jaws associated with the use of bisphosphonates. J Oral Maxillofac Surg 2004; 62: 527-534.
- 3. Marx RE. Pamidronate (Aredia) and Zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic [letter]. J Oral Maxillofac Surg 2003; 61: 115-118.
- 4. Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg 2003; 61: 1104-1107.
- 5. Ashcroft AJ, Davies FE, Morgan GJ. Aetiology of bone disease and the role of bisphosphonates in multiple myeloma. Lancet Oncol 2003; 4: 284-292.
- 6. Djulbegovic B, Whetley K, Ross J, et al. Bisphosphonates in multiple myeloma (Cochrane Review). Cochrane Database Syst Rev 2002 (3): CD003188.
- 7. Luckman SP, Hughes DE, Coxon FP, et al. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure activity relationships in J774 macrophages. J Bone Miner Res 1998; 13: 1668-1678.
- 8. Carter GD, Goss AN. Bisphosphonates and avascular necrosis of the jaws [letter]. Australian Prescriber 2004; 27: 32-33.
- 9. Carter GD, Goss AN. Bisphosphonates and avascular necrosis of the jaws [letter]. Aust Dent J 2003; 48: 268.
- 10. Adverse Drug Reactions Advisory Committee (ADRAC). Bisphosphonates and osteonecrosis of the jaw. Aust Adv Drug Reactions Bull 2005; 24: 3.