Most children with gastroenteritis do not develop dehydration and can be treated at home
Diarrhoea is defined as an increase in the frequency and fluidity of stools. Acute infectious diarrhoea (usually lasting less than 7 days) is a common reason for consulting a general practitioner and for admission to hospital in Australian children. Parents or carers consult a GP in up to 75% of cases of childhood gastroenteritis, while advice is sought from accident and emergency departments in about 50% and from pharmacists in about 5% of cases.1,2
In 1998–1999, the Australian Institute of Health and Welfare identified 22 709 children under the age of 15 years who were admitted to hospital with gastroenteritis, including three children under the age of 4 years who died of gastroenteritis.3 These figures underestimate the true burden of the disease in the community. A National Health Survey in 1995, using self-reported data, estimated that each year in the community there were around 4056 episodes of diarrhoea per 1000 children under 15 years.3
Case study — diarrhoea and vomiting in an infant
An 18-month-old girl, brought to the emergency department by her mother, has had eight watery green stools and five vomits in the previous 12 hours. She is eating poorly but drinks eagerly, has no fever and is bright and alert. Her eyes look sunken, but capillary return is normal. There is no blood in the stool and the vomitus is clear yellow in colour. She is an only child who attends long day-care, and has previously been well, with normal growth and development. The patient health record indicates she has lost 6% of body weight. Her mother, a nurse, is concerned that she might be dehydrated and asks, “Will she need to be admitted for a drip?”.
Management
The child has mild dehydration, as evidenced by the 6% weight loss and sunken eyes but normal capillary return. The clinical history of frequent vomiting and watery, non-bloody diarrhoea suggests a viral (probably rotavirus) infection.
The diagnosis of rotavirus infection is confirmed by stool immunofluorescence testing.
The mother agrees to try oral rehydration, and the child is offered frequent small sips of a commercial oral rehydration solution.
The vomiting settles rapidly, as is often the case with rotavirus infection, negating the need for intravenous fluids.
The child is discharged after 6 hours’ observation in hospital and the mother is advised to reintroduce the child’s usual diet as soon as this is tolerated.
Rotavirus infection is thought to account for half of all hospital admissions for severe diarrhoea.4 In New South Wales alone about 3700 children are admitted to hospital each year with rotavirus infection, at a cost to the health system of nearly $5 million.5 While the cost of hospitalisation for rotavirus infection is estimated at around $1700 per episode per child, the cost of care in the community (at $441 per child) is also significant. To both of these costing estimates must be added significant costs to the family, including time lost at work and missed schooling.6 Indigenous children have increased rates of gastroenteritis, more frequent and longer hospital admissions, and higher rates of dehydration, acidosis and comorbidity, than non-Indigenous children.7
Viral pathogens account for approximately 70% of episodes of acute infectious diarrhoea in children, and rotaviruses are most commonly implicated (Case study and Box 1). Bacterial infections account for about 15% of episodes and occur less often in developed than in developing communities. Overall, Campylobacter and Salmonella species are the most commonly reported bacterial infections in Australia, although atypical enteropathogenic Escherichia coli are emerging as important pathogens.8 Infections with Shiga-toxin-producing E. coli are rare in Australia, but may be complicated by the haemolytic–uraemic syndrome (HUS).9 Increasingly, E. coli infection, typhoid and shigellosis are reported in Australian children who have travelled abroad.
A history of contact with gastroenteritis can often be established in young children with diarrhoea and vomiting. However, diarrhoea and vomiting are non-specific symptoms in children and may also be associated with a range of infections outside the gastrointestinal tract (eg, otitis media, urinary tract infection), structural gut abnormalities (malrotation), metabolic diseases (diabetes mellitus), and surgical problems (acute appendicitis).
Children with viral gastroenteritis (usually due to rotavirus) generally present in autumn or winter with watery diarrhoea without blood, with or without vomiting, low-grade fever and anorexia. Most of these children are under the age of 5 years.
Children with bacterial gastroenteritis are more likely to have a high fever and blood and mucus in the stool.
A travel history should be sought in children with bloody diarrhoea. Haemolytic–uraemic syndrome (characterised by acute renal impairment, thrombocytopenia and microangiopathic haemolytic anaemia) should be considered in any child with bloody diarrhoea, pallor and poor urine output.
Organisms frequently implicated in food- or water-borne infections include species of Salmonella, Campylobacter, Listeria and Clostridia; Norwalk-like viruses (noroviruses); Shiga-toxin-producing Escherichia coli; and cryptosporidium. These infections are most commonly acquired from imported and takeaway foods, including seafood, red meat or poultry,10 and unpasteurised milk and contaminated water.
It is not feasible or necessary to take a stool specimen in all children with diarrhoea in the community. However, stool cultures may help guide treatment in very young, immunocompromised children or children with high fever who look particularly unwell; in children with gross bloody diarrhoea or a history of recent foreign travel; and in children who are part of an outbreak of diarrhoea in a child-care, school, community or hospital setting.
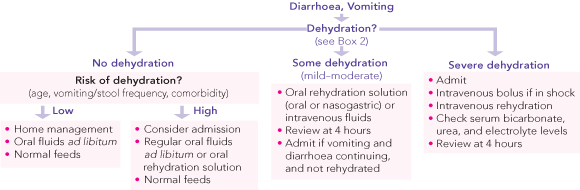
Children presenting with acute gastroenteritis should be assessed to determine the presence and severity of dehydration, as this will influence the choice of therapy. The likelihood of dehydration is increased in children with a history of frequent, severe watery diarrhoea and in children less than 6 months of age. Percentage weight loss gives the best estimate of the degree of dehydration, but this information is not always available and may not apply to all populations. For example, in a study of mainly Indigenous children presenting with acute gastroenteritis in Darwin, a low serum bicarbonate level correlated more strongly than weight change with the clinical assessment of the severity of dehydration.11
Clinical assessment of dehydration can be difficult, particularly in young infants, and rarely predicts the exact degree of dehydration accurately. Clinical signs will not be present until the child has lost at least 5% of body weight (ie, 5% dehydrated). Even trained medical staff overestimate the degree of dehydration,12 and this may result in unnecessary hospitalisation and treatment. The World Health Organization has simplified its classification of dehydration, and the clinical signs and percentage loss of body weight associated with different grades of dehydration (severe and mild to moderate) are shown in Box 2.13 A recent systematic review supports this classification, showing that prolonged capillary refill time, abnormal skin turgor and abnormal respiratory pattern were the three best clinical signs for identifying dehydration, whereas laboratory tests were often unhelpful and non-specific.14
In developed communities many non-dehydrated children without biochemical or acid–base disturbance are admitted to hospital, and many receive unnecessary intravenous (IV) fluids.1,2,12 The recommendations about which children with acute gastroenteritis should be admitted are based on consensus.15
Children without dehydration can be managed at home and should be offered their normal fluids ad libitum.
Children with mild to moderate dehydration should be observed in a paediatric facility for 4–6 hours to confirm that their oral intake is adequate and to determine the need for IV fluids and/or admission.
Children with shock or severe dehydration should be admitted for IV fluids.
A number of high-risk groups should be more readily considered for admission, including very young children, children with significant comorbidity, children in whom the diagnosis is not clear, and children whose parents are not able to care for them at home (Box 4). Studies from the United States suggest that admission rates are increased in families with poor social resources16 and where there is ready access to hospital services.17 There is a need for clinical trials to compare the relative risks and benefits of home-based and hospital-based management of children with mild dehydration.
Oral versus intravenous: Intravenous fluids are often used unnecessarily to treat dehydration associated with acute gastroenteritis.1,2,12 They are more expensive than oral fluids and their use requires admission to hospital. Electrolyte disturbance may result from the use of an incorrect type or volume of IV fluids. This may have severe consequences, including iatrogenic hyponatraemia which may result in brain injury or death. In addition, IV cannulation can be associated with emotional and physical trauma and infection. It is therefore reasonable to question whether oral rehydration solutions (ORS) are just as effective as IV fluids for managing children with mild to moderate dehydration.
In one systematic review, IV and oral rehydration therapies were compared in children with mild to moderate dehydration. No significant differences were found between IV and oral therapy with regard to duration of diarrhoea, time spent in hospital and weight at discharge from hospital.18 A more recent systematic review of 16 randomised controlled trials (RCTs) (1545 children with acute gastroenteritis) confirmed these findings.19 Five trials in this review included children with severe dehydration and two included malnourished children. The meta-analysis showed that children treated with enteral rehydration (oral or nasogastric) had significantly lower rates of death and/or seizures (relative risk, 0.36; 95% CI, 0.14–0.89) and a significantly shorter hospital stay (mean difference, 21 hours; 95% CI, 8–35 hours) than children who received IV fluids. Weight gain in the two groups was similar, and the overall failure rate of enteral therapy (need for unscheduled IV fluids) was only 4%.19
Two RCTs compared nasogastric administration of ORS with IV rehydration and showed that nasogastric administration of rehydration fluids is as safe and effective as IV rehydration in acute infectious diarrhoea with mild to moderate dehydration.20,21 Nasogastric fluid administration was well tolerated and no adverse effects were reported in either study. Infiltration at the site of cannulation was the most common problem associated with IV rehydration.20
Oral/nasogastric rehydration issues: Some children refuse ORS because they dislike the taste. However, the addition of cordial and other sweeteners should be discouraged, because these increase the osmolarity of ORS and may worsen the diarrhoea. Children with significant dehydration will usually drink readily. In rotavirus infection, vomiting usually occurs early in the illness and settles as diarrhoea increases. Most of these children tolerate frequent small sips of ORS. In a child with profuse ongoing vomiting, ORS can be given via a nasogastric tube.
Rapid rehydration: Rehydration over approximately 4 hours has recently gained acceptance in developing countries22 and may reduce the need for admission to hospital. In one prospective study using historical controls, children given ORS via a nasogastric tube or IV fluids administered at a rate of 20 mL/kg per hour had decreased hospital admission rates.23 No adverse events were reported in this study. However, the intravenous fluids used for rapid rehydration must be selected carefully to prevent iatrogenic hyponatraemia. Fluids such as Hartmann’s solution, normal saline or N/2 saline should be used rather than hypotonic maintenance solutions, such as N/4 saline. (N/2 saline and N/4 saline have half and a quarter, respectively, the sodium chloride concentration of normal saline.) In Aboriginal children hospitalised in the Northern Territory rapid intravenous rehydration is used for moderate to severe dehydration.11
Low-osmolarity ORS: A hypotonic ORS is preferred for children with acute diarrhoea and dehydration in developed communities. A systematic review of ORS formulations with osmolarity 200–250 mOsm/L versus higher-osmolarity solutions (WHO standard ORS) found that they decrease stool output and vomiting risk and the need for unscheduled IV fluids.24 The composition of ORSs commercially available in Australia is shown in Box 5.
Cereal-based ORS: In developing communities, where recurrent episodes of infectious diarrhoea contribute to malnutrition, the use of a food (cereal)-based ORS minimises the impact of infection on nutrition. Because these ORS formulations contain complex carbohydrates, they also have a lower osmotic penalty than glucose–electrolyte solutions. A systematic review comparing the efficacy of cereal-based versus glucose-based WHO rehydration solutions in children hospitalised with acute gastroenteritis found that, in children with cholera-like diarrhoea, the cereal-based solution significantly lowered stool output at 24 hours compared with the WHO-ORS.25 In the only RCT conducted in a developed community in young children,26 a rice-based ORS was compared with a glucose-based solution with a similar electrolyte composition. The cereal-based solution significantly decreased mean stool volume, duration of diarrhoea (but not hospitalisation) and time to resumption of normal diet and fluids in young children hospitalised with acute gastroenteritis and mild to moderate dehydration. There were no adverse effects and both solutions were well tolerated.
There are no published trials comparing clear fluids (water, carbonated drinks, fruit drinks) with glucose–electrolyte solutions for the treatment of mild to moderate dehydration. However, physiological studies have shown that these drinks (which are low in sodium and potassium and have a high sugar content and high osmolarity) may exacerbate diarrhoea and dehydration and cause electrolyte disturbance. Thus, their use is not recommended in children with significant dehydration.13,15,22
Historically, the management of acute gastroenteritis included a period of fasting. However, clinical studies have shown that an early return to feeding has both clinical and nutritional benefits.27 A meta-analysis of RCTs showed that early refeeding in gastroenteritis reduces the duration of diarrhoea (0.43 days; 95% CI, − 0.74 to 0.12).22 Two subsequent RCTs showed that early refeeding improves weight gain without increasing either vomiting or diarrhoea duration.28,29 Clinical practice guidelines from the American Academy of Pediatrics22 and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition30 recommend early refeeding for the management of gastroenteritis in children. Complex carbohydrates, lean meats, yogurt, fruits and vegetables should be given, while foods high in fat and simple sugars should be avoided.22 Breastfeeding should be continued during episodes of acute diarrhoea.15,22,30,31 Dilution of formula is not necessary when it is reintroduced into the diet of formula-fed infants.22,30
Antibiotic and antiemetic agents are frequently prescribed for acute diarrhoea,1,2 but there is little evidence of their effectiveness and they may have adverse effects. Antibiotics may lead to antimicrobial resistance and should be reserved for children with invasive bacterial infections — giardiasis, shigella and cholera. Treatment of Shiga-toxin-producing E. coli with antibiotics or antimotility agents increases the risk of haemolytic–uraemic syndrome. Antiemetics have no proven benefit in acute gastroenteritis and may result in adverse effects, including acute dystonic reactions in children.
Antimotility agents such as loperamide (an opiate) may reduce the duration of diarrhoea in children, but are not recommended for children aged under 12 years. There are concerns regarding reported adverse effects, which include lethargy, ileus, respiratory depression, coma and death.22 The use of loperamide in children with acute diarrhoea has been evaluated in five RCTs.32 In two of the three trials reporting effect on duration of diarrhoea, taking loperamide (compared with placebo) significantly reduced diarrhoea duration. There was no significant difference in the groups receiving loperamide or placebo in the duration of hospital stay (reported in three RCTs). Weight gain at 3 days was better in children receiving loperamide than in the group receiving placebo (two of the three trials reporting weight gain). Four of the five RCTs reported no adverse effects from loperamide. In the remaining trial, an increased rate of adverse events (including abdominal distension and lethargy) occurred in children receiving loperamide, and the rates of adverse events were higher in those receiving the higher (0.8 mg/kg) compared with the lower (0.4 mg/kg) dose of loperamide.
Damage to the small intestinal mucosa by pathogens causing acute gastroenteritis may result in temporary lactose intolerance. In the child with prolonged watery diarrhoea (> 7 days) associated with perianal excoriation, carbohydrate malabsorption should be excluded by testing the stool for reducing substances and, if confirmed, lactose-free feeds may be indicated.
But should lactose-free feeds be used routinely in the initial management of gastroenteritis to reduce the duration of symptoms? In a systematic review33 of studies comparing lactose-free and lactose-containing diets for children with diarrhoea and mild to moderate dehydration, lactose-free diets appeared to decrease the duration of diarrhoea in some subgroups of children. The significant heterogeneity between these studies brings into question the validity of pooling these data. Of five RCTs published subsequently,32 three small studies reported no difference in diarrhoea duration between groups. In the two larger trials children receiving lactose-free feeds had reduced duration of diarrhoea compared with children receiving lactose-containing feeds. Thus, there is some evidence that a lactose-free diet may reduce the duration of diarrhoea in children with acute gastroenteritis. It is difficult to make a general recommendation regarding this question owing to the significant variability in results of RCTs.
Gastroenteritis, predominantly caused by rotavirus, remains a major cause of morbidity in young children. The approach to prevention in the community must be multipronged and should include licensing of new vaccines (including a rotavirus vaccine), educating people about personal and food hygiene, and encouraging breastfeeding. Probiotics may prove useful both for preventing infectious diarrhoea and as an adjunct to oral rehydration therapy.34 Future development of safe antimotility and antisecretory agents may also be valuable to reduce diarrhoeal losses in some children. However, the immediate challenge is to increase the use of treatments of proven efficacy — including ORSs — and to minimise the risk of iatrogenic harm from unnecessary use of intravenous fluids, antibiotics, and antidiarrhoeal and antiemetic agents.
Evidence-based practice tips
Rehydration
Intravenous fluids are recommended for children with shock and/or severe dehydration. Oral rehydration is the treatment of choice in mild to moderate dehydration — it is equally effective and has fewer adverse effects than intravenous therapy (I).18,19
Nasogastric administration of an oral rehydration solution is as effective as intravenous fluids for mild to moderate dehydration and is well tolerated (II).20,21
Refeeding
Early refeeding improves weight gain without increasing diarrhoea or vomiting. Children with acute gastroenteritis should not be fasted and should be offered their usual diet after a 4–6-hour period of rehydration (I).22
Oral rehydration solutions
Hypotonic oral rehydration solutions (approximately 240 mOsm/L), such as those commercially available in Australia, should be used to treat mild to moderate dehydration (I).24
Cereal-based oral rehydration solutions are of proven efficacy in cholera-like diarrhoea and have nutritional benefits (I).25
Limited evidence from developed communities suggests rice-based solutions are as effective as, and may have advantages over, glucose-based rehydration solutions (II).26
Scientifically formulated glucose–electrolyte solutions should be used to treat children with acute infectious diarrhoea and mild to moderate dehydration and ongoing diarrhoea and vomiting (II).18,22
Antimotility agents
Loperamide may reduce the duration of diarrhoea in children with acute gastroenteritis (II).32 However, this therapy is not recommended for routine use in acute infectious diarrhoea, because the illness is self-limiting and the potential risks outweigh the benefits (IV).22
Lactose-free feeds
Routine use of lactose-free feeds is not recommended (I).33
Levels of evidence (I–IV) are derived from the National Health and Medical Research Council’s system for assessing evidence.35
1 Causes of acute gastroenteritis in children*
Viruses (~70%)
|
Bacteria (~15%)
|
||||||||||||||
Protozoa
|
Helminths
|
||||||||||||||
* Listed in approximate order of frequency. † Notifiable in all jurisdictions except New South Wales. ‡ Notifiable disease. Outbreaks of gastroenteritis in institutions are also notifiable. |
|||||||||||||||
2 Assessment of dehydration in children
No dehydration |
Mild–moderate dehydration |
Severe dehydration |
|||||||||||||
Body weight |
|||||||||||||||
No loss of body weight |
Loss of body weight: |
Loss of body weight: > 10% |
|||||||||||||
Clinical signs |
|||||||||||||||
None |
Two or more of:* |
Two or more of:* |
|||||||||||||
|
|
|
|||||||||||||
Pinch test (measuring skin turgor)† |
|||||||||||||||
Normal: skin fold retracts immediately |
Slow: skin fold visible < 2 seconds |
Very slow: skin fold visible > 2 seconds |
|||||||||||||
* Additional signs of severe dehydration include circulatory collapse (eg, weak rapid pulse, cool or blue extremities or hypotension), rapid breathing, or sunken anterior fontanelle. † Skin turgor is assessed by pinching the skin of the abdomen or thigh between the thumb and the bent forefinger in a longitudinal manner. The sign is unreliable in obese or severely malnourished children. |
|||||||||||||||
4 When to admit children with diarrhoea and vomiting
Children should be admitted to hospital if they are:
Mildly to moderately dehydrated — observe for 4–6 hours to ensure adequate rehydration;
Severely dehydrated or in shock — intravenous fluids are required;
At high risk of dehydration — < 6 months old, high frequency of watery stools or vomits, minimal oral intake, worsening symptoms;
At high risk of complications — children with significant underlying disease (eg, diabetes, renal failure), high fever, poor nutrition; or
Suspected of having another diagnosis (eg, appendicitis, intussusception).
Children should also be admitted if the parent or carer is unable to manage the child at home.
5 Oral rehydration solutions commercially available in Australia
Name |
Glucose (mmol/L) |
Sodium (mmol/L) |
Chloride (mmol/L) |
Potassium (mmol/L) |
Base (mmol/L) |
Osmolarity (mOsm/L) |
|||||||||
Glucose–electrolyte solutions |
|||||||||||||||
Gastrolyte |
90 |
60 |
60 |
20 |
Citrate 10 |
240 |
|||||||||
Hydralyte |
90 |
45 |
45 |
20 |
Citrate 30 |
240 |
|||||||||
Pedialyte |
126 |
45 |
35 |
20 |
Citrate 10 |
246 |
|||||||||
Repalyte new formulation |
90 |
60 |
60 |
20 |
Citrate 10 |
240 |
|||||||||
Rice-based electrolyte solutions |
|||||||||||||||
Gastrolyte-R |
6 g pre-cooked rice/L |
60 |
50 |
20 |
Citrate 10 |
226 |
|||||||||
European Society of Paediatric Gastroenterology, Hepatology and Nutrition recommendation.30 |
|||||||||||||||
|
74–111 |
60 |
Not < 30 |
20 |
Citrate 10 |
200–250 |
|||||||||
- 1. Elliott EJ, Backhouse JA, Leach JW. Pre-admission management of acute gastroenteritis. J Paediatr Child Health 1996; 32: 18-21.
- 2. O’Loughlin EV, Notaras E, McCullough C, et al. Home-based management of children hospitalized with acute gastroenteritis. J Paediatr Child Health 1995; 31: 189-191.
- 3. Mathers C. Diarrhoeal diseases and gastroenteritis: Australia. Australian Institute of Health and Welfare. Burden of disease and disease expenditure. Available at: www.aihw.gov.au/bod/bod_yld_by_disease/index.html (via a linked spreadsheet — A4 Diarrhoea <www.aihw.gov.au/bod/bod_yld_by_disease/a_infectious/a4_diarrhoea.xls>) (accessed May 2004).
- 4. Carlin JB, Chondros P, Masendycz P, et al. Rotavirus infection and rates of hospitalisation for acute gastroenteritis in young children in Australia, 1993-1996. Med J Aust 1998; 169: 252-256. <MJA full text>
- 5. Ferson MJ. Hospitalisations for rotavirus gastroenteritis among children under five years of age in New South Wales. Med J Aust 1996; 164: 273-276.
- 6. Liddle JL, Burgess MA, Gilbert GL, et al. Rotavirus gastroenteritis: impact on young children, their families and the health care system. Med J Aust 1997; 167: 304-307.
- 7. Ruben AR, Fisher DA. The casemix system of hospital funding can further disadvantage Aboriginal children. Med J Aust 1998; 169 (8 Suppl): S6-S10. <MJA full text>
- 8. Robins-Browne RM, Bordun AM, Tauschek M, et al. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg Infect Dis 2004; 10: 1797-1805.
- 9. Elliott EJ, Robins-Browne RM, O’Loughlin EV, et al. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological and epidemiological features. Arch Dis Child 2001; 85: 125-131.
- 10. Ashbolt R, Givney R, Gregory JE, et al. Enhancing foodborne disease surveillance across Australia in 2001: the OzFoodNet Working Group. Commun Dis Intell 2002; 26: 375-406.
- 11. Brewster D. Dehydration in acute gastroenteritis. J Paediatr Child Health 2002; 38: 219-222.
- 12. Mackenzie A, Barnes G, Shann F. Clinical signs of dehydration in children. Lancet 1989; 2: 605-607.
- 13. World Health Organization. Child and adolescent health and development. Assessing the diarrhoea patient. Available at: www.who.int/child-adolescent-health/New_Publications/CHILD_HEALTH/Meded/3med.htm (accessed Apr 2004).
- 14. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA 2004; 291: 2746-2754.
- 15. Armon K, Stephenson T, MacFaul R, et al. An evidence and consensus based guideline for acute diarrhoea management. Arch Dis Child 2001; 85: 132-142.
- 16. Fitzgerald M, McGee HM. Psychological health status of mothers and the admission of children to hospital for gastroenteritis. Fam Pract 1990; 7: 116-120.
- 17. Goodman DC, Fisher ES, Gittelsohn A, et al. Why are children hospitalized? The role of non-clinical factors in pediatric hospitalizations. Pediatrics 1994; 93: 869-902.
- 18. Gavin N, Merrick N, Davidson B. Efficacy of glucose-based oral rehydration therapy. Pediatrics 1996; 98: 45-51.
- 19. Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis: a meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med 2004; 158: 483-490.
- 20. Gremse DA. Effectiveness of nasogastric rehydration in hospitalized children with acute diarrhea. J Pediatr Gastroenterol Nutr 1995; 21: 145-148.
- 21. Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics 2002; 109: 566-572.
- 22. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996; 97: 424-435.
- 23. Phin SJ, McCaskill ME, Browne GJ, Lam LT. Clinical pathway using rapid rehydration for children with gastroenteritis. J Paediatr Child Health 2003; 39: 343-348.
- 24. Hahn S, Kim S, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children (Cochrane Review). The Cochrane Library. Issue 2, 2004. Chichester, UK: John Wiley & Sons.
- 25. Fontaine O, Gore S, Pierce NF. Rice-based oral rehydration solution for treating diarrhoea. The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley and Sons.
- 26. Wall CR, Swanson CE, Cleghorn GJ. A controlled trial comparing the efficacy of rice-based and hypotonic oral rehydration solutions in infants and young children with gastroenteritis. J Gastroenterol Hepatol 1997; 12: 24-28.
- 27. Sandhu BK; European Society of Paediatric Gastroenterology, Hepatology and Nutrition Working Group on Acute Diarrhoea. Rationale for early feeding in childhood gastroenteritis. J Pediatr Gastroenterol Nutr 2001; 33: S13-S16.
- 28. Brown KH, Gastanaduy AS, Saavedra JM, et al. Effect of continued oral feeding on clinical and nutritional outcomes of acute diarrhea in children. J Pediatr 1988; 112: 191-200.
- 29. Sandhu BK, Isolauri E, Walker-Smith JA, et al. A multicentre study on behalf of the European Society of Paediatric Gastroenterology and Nutrition Working Group on Acute Diarrhoea. Early feeding in childhood gastroenteritis. J Pediatr Gastroenterol. Nutr 1997; 24: 522-527.
- 30. Sandhu B; European Society of Paediatric Gastroenterology, Hepatology and Nutrition Working Group on Acute Diarrhoea. Practical guidelines for the management of gastroenteritis in children. J Pediatr Gastroenterol Nutr 2001; 33: S36-S39.
- 31. Khin MU, Nyunt-Nyunt-Wai, Myo-Khin, et al. Effect on clinical outcome of breast feeding during acute diarrhoea. BMJ 1985; 290: 587-589.
- 32. Dalby-Payne J, Elliott E. Gastroenteritis in children. Clin Evid 2003; 9: 367-376.
- 33. Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics 1994; 93: 17-27.
- 34. Allen SJ, Okoko B, Martinez E, et al. Probiotics for treating infectious diarrhoea (Cochrane Review). The Cochrane Library, Issue 2, 2004. Chichester, UK: John Wiley & Sons.
- 35. National Health and Medical Research Council. How to use the evidence: assessment and application of scientific evidence. Handbook series on preparing clinical practice guidelines. Table 1.3: Designation of levels of evidence. Canberra: NHMRC, February 2000: 8. Available at: www.health.gov.au/nhmrc/publications/pdf/cp69.pdf (accessed Sep 2004).






Abstract
Gastroenteritis in children is still a common reason for consulting a general practitioner and for hospital admission.
Rotavirus is the most common cause of gastroenteritis in children and accounts for half of all hospital admissions for severe acute infectious diarrhoea.
Most children with gastroenteritis do not develop dehydration and can be treated at home.
Children with mild to moderate dehydration should be treated with low osmolarity oral rehydration solutions, and those with severe dehydration or shock need to be admitted for administration of intravenous fluids.
Lactose-free feeds should not be routinely used after acute gastroenteritis, but there is some evidence that a lactose-free diet may reduce the duration of diarrhoea.
Antimotility drugs are rarely indicated in children with gastroenteritis, as the potential risks outweigh the benefits.
The development of a rotavirus vaccine would provide huge public health benefits and cost savings. Other preventive strategies include educating people about personal and food hygiene and encouraging breastfeeding.