Peripheral arterial disease, whether symptomatic or not, refers to occlusive disease of lower-limb arteries. It is most commonly caused by atherothrombosis, but may reflect other disease, such as arteritis, aneurysm, and embolism. In recent years, it has become evident that PAD is an important predictor of substantial coronary and cerebral vascular risk.1-4
Increased awareness of the prognostic importance of PAD5,6 has led to a search for sensitive diagnostic markers. The ankle–brachial pressure index (ABPI) has emerged as a valid and reliable marker of PAD and its attendant vascular risk, particularly in patients without clinical features of PAD.7,8 However, because awareness and implementation of the ABPI in general clinical practice is poor, the concept and prevalence of asymptomatic PAD is not widely appreciated, and PAD continues to be underdiagnosed and undertreated.5 There is also evidence that the management of risk factors in patients with symptomatic PAD is inadequate.9
In this article, we aim to examine:
ABPI as a valid and reliable diagnostic marker of PAD;
PAD, defined either by symptoms or an abnormal ABPI, as a significant independent risk factor for systemic atherothrombosis;
incorporating an assessment of PAD into the assessment of overall vascular risk; and
modifiable causal risk factors for PAD, and the potential for appropriate vascular risk factor control to reduce the burden of vascular disease.
We will not discuss the management of the lower-limb consequences of PAD.
Most clinicians think of PAD in terms of symptoms (intermittent claudication, rest pain) and abnormal signs (diminished peripheral pulses, ischaemic ulceration and gangrene). The clinical diagnosis and assessment of severity of intermittent claudication is not always reliable, and various structured questionnaires (notably World Health Organization/Rose and Edinburgh Claudication questionnaires) have been used in epidemiological studies.10 However, PAD can be confirmed in most cases by measuring the ABPI (Box 1).11,12 This index is a simple, non-invasive bedside tool for diagnosing PAD that can be used by any clinician. An ABPI of less than 0.9 is diagnostic of PAD (Box 2). In addition to providing a semi-quantitative and objective measure of the severity of symptomatic PAD, the index also allows for identification of asymptomatic PAD (Box 3). The validity of the ABPI as a diagnostic marker of subclinical arterial vascular disease is confirmed by its adverse prognostic significance for coronary and cerebrovascular events.1,2,7,8
Duplex scanning, magnetic resonance imaging, computed tomography and digital subtraction angiography are also useful in assessing PAD, but are generally used for anatomical localisation of arterial disease before intervention rather than for initial diagnosis.
The incidence of symptomatic PAD increases with age, from about 0.3% per year for men aged 40–55 years to about 1% per year for men aged over 75 years.10 The prevalence of PAD varies considerably depending on how PAD is defined, and the age (and, to a lesser extent, sex) of the population being studied.14 Using the definition of an ABPI less than 0.9, most epidemiological studies report the prevalence of PAD to be about 10%–25% in men and women over 55 years of age.15 Although only about 10%–20% of people with PAD identified in epidemiological studies are symptomatic (usually with intermittent claudication), this may be an underestimate because of underascertainment of symptomatic cases.15,16 The prevalence of PAD rises with age (eg, from 10.6% in men aged 65–69 years to 23.3% in men aged 75–79 years in a population-based Western Australian study17). On average, the prevalence of symptomatic disease at around 60 years of age is about 5%.10
A minority of patients with intermittent claudication suffer from worsening leg symptoms (rest pain, ischaemic ulceration or gangrene). Estimates of the proportion of patients with intermittent claudication needing intervention, such as angioplasty or bypass surgery, vary considerably, but are of the order of 10%–20%, with only 1%–2% of patients progressing to amputation over five years.10 Continued smoking, diabetes and a low initial ABPI are the main risk factors for progression to amputation.10
While most patients fear amputation, it is cardiac or cerebrovascular events that are the major threats to handicap-free survival. PAD is associated with a fourfold increase in the risk of cardiovascular death, from about 1% per year among control subjects to 4%–6% per year among patients with PAD.3,8,18 The more severe the PAD as measured by the ABPI, the worse the prognosis (Box 4).4,8,12 Patients with symptomatic PAD have a 15-year accrued survival rate of about 22%, compared with a survival rate of 78% in patients without symptoms of PAD. Patients with critical leg ischaemia, who have the lowest ABPI values, have an annual mortality of 25%.19
Much of the evidence pertaining to primary and secondary prevention of cardiovascular events in patients with PAD has been extrapolated from observational and case–control studies of patients with PAD, and randomised-controlled trials in patients with coronary heart disease. The evidence we discuss here for the use of interventions is graded according to the National Health and Medical Research Council system for assessing levels of evidence.20 Unfortunately, not all of the interventions known to reduce cardiovascular mortality (Box 5) will necessarily improve the symptoms of intermittent claudication.
Smoking cessation remains a priority for all smokers — the survival benefit from cessation is unequivocal.21 Over 80% of patients with PAD are current or ex-smokers.19 The association between smoking and PAD is about twice as strong as that with coronary heart disease.10,22 Smoking results in earlier onset of symptoms and the severity of PAD increases with the number of cigarettes smoked. For patients with intermittent claudication, previous smoking has a long legacy of increased risk of PAD.17,18,22 It is important to emphasise to patients with symptomatic PAD that their leg symptoms will probably worsen, the results of any intervention will be less successful and the risks of amputation greater if they continue to smoke.23 Strategies for helping patients quit smoking have been reviewed elsewhere.23
There is ample evidence that regular exercise is good for cardiovascular health.24 There is also evidence that exercise can improve walking distance in patients with intermittent claudication.25 It is likely that a structured or supervised program is most effective, particularly for older patients. Weight loss is generally associated with improved cardiovascular health and, in the case of intermittent claudication, it should, for obvious reasons, result in an increased walking distance.
The association between hyperlipidaemia and PAD is not as strong as it is with coronary heart disease. There is, however, recent evidence that lipid-lowering with a statin may improve walking distance in patients with intermittent claudication.28 More importantly, the Heart Protection Study reported that simvastatin (40 mg daily) use in patients with PAD (including those without prior coronary heart disease) resulted in a highly significant 25% proportional reduction in all major vascular events.29 For this reason alone, a statin should be considered for all patients with PAD, although the threshold cholesterol level for qualifying for a Pharmaceutical Benefits Scheme authority prescription is still higher for PAD than coronary heart disease (> 6.5 mmol/L v 4.0 mmol/L30).
Intervention trials in hypertension have not included intermittent claudication as an endpoint.31 It is unlikely that controlling hypertension will improve intermittent claudication, but it will reduce the risk of other cardiovascular events, particularly stroke.32
Diabetes is important in PAD for a number of reasons (Box 6). Patients with diabetes are prone to medial calcification (Mönckeberg’s sclerosis) of lower-limb arteries in particular. While not an occlusive process, this pattern of calcification is a marker of poor prognosis.33 Good glycaemic control has the potential to prevent, or at least delay, amputation in patients with diabetes. This is primarily through preventing neuropathy and its associated ulceration and infection rather than stopping progression of any macrovascular PAD. Intensive glycaemic control also reduces the risk of other diabetes-related microvascular disease, particularly renal and ocular complications.34
Aspirin has an established role in preventing myocardial infarction, stroke and cardiovascular death.35 Aspirin does not improve intermittent claudication, but appears to delay progression and reduce the need for intervention in PAD.36 In terms of secondary prevention, the CAPRIE study showed that clopidogrel compared with aspirin was associated with an 8.7% (95% CI, 0.3%–16.5%) relative-risk reduction in stroke, myocardial infarction, or vascular death.37 Subgroup analyses suggested that the greatest efficacy of clopidogrel compared with aspirin was in patients with PAD, in whom there was a 24% risk reduction. Despite these data, clopidogrel is not approved as a replacement for aspirin in patients who have worsening PAD despite aspirin therapy.30 Warfarin is not indicated for uncomplicated PAD, although it may be useful following intervention.
Several large trials have shown that ACE inhibitors improve outcomes in patients with cardiovascular disease,38 including those with clinical or subclinical PAD.39 The magnitude of the treatment effect was greater than might be expected by the reduction achieved in blood pressure, suggesting that ACE inhibitors may have a direct beneficial effect on the heart or vasculature independent of their effect on blood pressure. Of interest is that subgroup analysis in the HOPE trial suggested that patients with PAD gained even more benefit from ramipril than those without PAD.38 Although ACE inhibitors are of potential concern in renal artery stenosis (which is associated with PAD), it is likely that, with careful monitoring, the secondary prevention benefits of ACE inhibitors outweigh the risks in patients with PAD.31
This topic has been reviewed elsewhere.12 Numerous drugs for treating intermittent claudication have been studied in randomised and non-randomised trials; most were too small to be conclusive. The drug with the most promise at present is cilostazol (an inhibitor of phosphodiesterase type 3), which has been shown to significantly improve walking distance by 20%–40% in four trials involving 1534 patients with intermittent claudication12 (E1). This drug is not currently available in Australia.
Elevated blood levels of homocysteine, C-reactive protein and fibrinogen have all been reported in patients with PAD, but it remains to be shown that they are both causal and modifiable risk factors for atherothrombosis. Modest alcohol consumption may be beneficial in patients with PAD.10
The “conservative therapy” approach to intermittent claudication (let alone asymptomatic PAD) practised by many clinicians, particularly vascular surgeons, has tended to consist of reassurance, surveillance and half-hearted strategies for promoting exercise and smoking cessation.31 This approach is inadequate; there is now compelling evidence that patients with PAD are at high risk of cardiovascular death and that this risk can be reduced by a range of medical therapies. The ABPI is an independent predictor of coronary and cerebrovascular morbidity and mortality, and provides a convenient tool for further stratifying patients according to their cardiovascular risk. This is particularly so for those determined to be at intermediate risk by traditional Framingham-based criteria, who may not otherwise qualify for the most intensive risk-factor intervention.40 Data from several large drug trials indicate that patients with PAD may gain as much, if not more, benefit from intervention than those with other manifestations of atherothrombosis.29,37,38 Although specialist vascular surgeons should be involved in managing severe PAD (Box 7), management of mild disease and risk factors should be undertaken by all clinicians.
From a population perspective, secondary prevention has its limitations, as most vascular events occur in individuals without pre-existing clinical disease. Screening for asymptomatic atherothrombotic disease using the ABPI, followed by primary-prevention measures, has been advocated in the past.41 This is supported by the observation that people with asymptomatic PAD appear to have the same increased risk of cardiovascular death as those with intermittent claudication, and that some of these deaths are preventable.42 However, several questions need to be considered before implementing routine screening for risk factors in clinical practice,43 and many of these remain unanswered with respect to the ABPI (Box 8). We believe it is premature to recommend screening for PAD in the general population, as there are as yet insufficient data to support the pharmacological treatment of asymptomatic PAD in patients without other cardiovascular risk factors, and it remains to be shown that the overall benefit outweighs the adverse consequences and costs associated with screening and follow-up.
The introduction of similar regulations for the prescribing of statins and clopidogrel for symptomatic PAD as for coronary heart and cerebrovascular disease also needs to be addressed.
1 Measuring the ankle–brachial pressure index
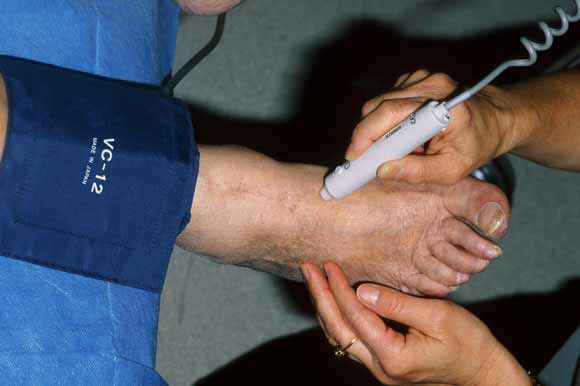
(a) Hand-held Doppler probe being used to measure systolic pressure in the dorsalis pedis pulse.
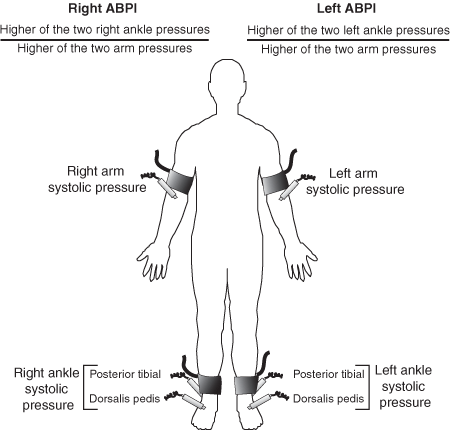
(b) Measurement of the ankle–brachial pressure index.
ABPI = ankle–brachial pressure index. Adapted from Hiatt WR.11
2 The ankle–brachial pressure index*
Ankle–brachial pressure index* |
Interpretation† |
||||||||||||||
> 1.1 |
Normal but consider incompressible (calcified) arteries |
||||||||||||||
0.9–1.1 |
Normal range |
||||||||||||||
0.7–0.89 |
Consistent with mild to moderate peripheral arterial disease (asymptomatic or intermittent claudication) |
||||||||||||||
< 0.7 |
Consistent with moderate to severe peripheral arterial disease (intermittent claudication or rest pain) |
||||||||||||||
* The ratio of the brachial systolic pressure divided by the systolic pressure detected in the posterior tibial or dorsalis pedis pulse (whichever is highest), measured as shown in Box 1. † Based on Fowkes.13 |
|||||||||||||||
3 The role of ankle–brachial pressure index in clinical practice
Confirms the clinical diagnosis of peripheral arterial disease, particularly in patients with atypical symptoms or co-existing musculoskeletal problems.
Provides a semi-quantitative (see Box 2) assessment of:
severity of peripheral arterial disease, including asymptomatic disease;
progression of peripheral arterial disease over time; and
response to intervention for peripheral arterial disease.
Provides an indication of overall cardiovascular risk (see Box 4).
4 Kaplan–Meier survival plot for 4268 patients at risk for incident cardiovascular disease, by level of ankle–brachial pressure index
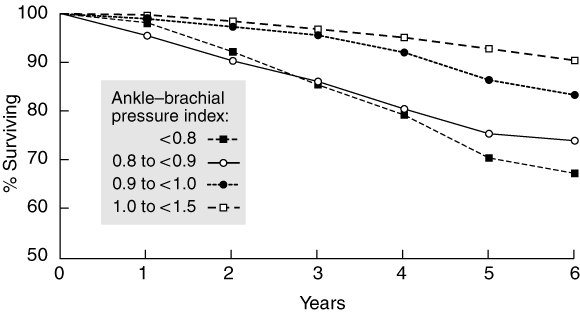
Reproduced with permission from Newman et al.12 Ankle–brachial pressure index (ABPI) < 0.8, 213 patients; ABPI 0.8 to < 0.9, 196 patients; ABPI 0.9 to <1.0, 536 patients; ABPI 1.0 to < 1.5, 3323 patients. An ABPI less than 0.9 was associated with a 1.62 (95% CI, 1.24–2.12) relative risk of death.
5 Strategies for secondary prevention of serious vascular events in patients with peripheral arterial disease*
Smoking cessation (E32)
Regular exercise and weight loss (E32)
Treatment of diabetes (E2)
Treatment of hypertension (E2)
Treatment of hyperlipidaemia with statins (E2)
Antiplatelet drug therapy (E1)
Angiotensin-converting enzyme inhibitor (E2)
* See reference 20 for definition of levels of evidence.
6 Diabetes and peripheral arterial disease
The prevalence of diabetes in intermittent claudication is up to 20%, with some cases of diabetes previously undiagnosed (therefore, consider screening for diabetes).
Intermittent claudication is about twice as common in patients who have diabetes compared with patients who do not.
Neuropathy associated with diabetes greatly increases the risk of foot sepsis and progression to amputation; more intensive management of all risk factors in patients with intermittent claudication and diabetes is indicated.
Ankle–brachial pressure index may be spuriously high in patients with diabetes because arteries are incompressible (calcified).
7 Indications for referring a patient to a vascular surgeon
Rest pain, ischaemic ulceration or gangrene.
Intermittent claudication which interferes with work or lifestyle, has not responded to a trial of non-interventional management, or is associated with diabetes.
Diagnostic uncertainty: atypical symptoms, coexisting musculoskeletal cause for pain, ambiguous ankle–brachial pressure index (incompressible arteries).
Other (eg, significant carotid stenosis or aneurysmal disease).
8 Questions that need to be answered before the ankle–brachial pressure index (ABPI) can be recommended for population screening*
Answer is yes or probably yes:
1. Is ABPI a convenient, standardised and valid test that independently predicts events?
2. Are there population norms to guide the interpretation of the results of ABPI?
3. Is a low ABPI specific for increased cardiovascular risk?
4. Does measuring the ABPI provide clinically significant prognostic value above and beyond that provided by traditional risk factors?
Answer is unknown:
5. Will the results of the ABPI change clinical management?
6. What are the direct and indirect risks of screening (eg, false-positive and false-negative results)?
7. Does modification of the treatment plan on the basis of the results of the ABPI lead to clinical benefit?
8. Does the overall benefit of measuring ABPI outweigh the adverse consequences and costs associated with screening and follow-up?
* Adapted from Mosca.43
- 1. Ogren M, Hedblad B, Isacsson S, et al. Ten year cerebrovascular morbidity and mortality in 68 year old men with asymptomatic carotid stenosis. BMJ 1995; 310: 1294-1298.
- 2. Tsai A, Folsom A, Rosamond W, Jones D. Ankle–brachial index and 7-year ischemic stroke incidence. The ARIC Study. Stroke 2001; 32: 1721-1724.
- 3. Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J 1994; 72: 128-132.
- 4. Newman A, Shemanski L, Manolio T, et al. Ankle–arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1999; 19: 538-545.
- 5. Hirsch A, Criqui M, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317-1324.
- 6. Newman AB, Arnold AM, Naydeck BL, et al. “Successful Aging”. Effect of subclinical cardiovascular disease. Arch Intern Med 2003; 163: 2315-2322.
- 7. Zheng Z, Sharrett A, Chambless LE, et al. Associations of ankle–brachial index with clinical coronary heart disease, stroke, and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 1997; 131: 115-125.
- 8. Leng GC, Fowkes FGR, Lee AJ, et al. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ 1996; 313: 1440-1444.
- 9. Teh L, Sieunarine K, Eikelboom J, et al. Suboptimal preventive practices in patients with carotid and peripheral vascular occlusive disease in a tertiary referral setting. ANZ J Surg 2003; 73: 932-937.
- 10. Trans Atlantic Inter-Society Consensus (TASC) on the management of peripheral arterial disease. Eur J Vasc Endovasc Surg 2000; 19(Suppl A): S1-S244.
- 11. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608-1621.
- 12. Newman AB, Shemanski L, Manolio TA, et al. Ankle–arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1999; 19: 538-545.
- 13. Fowkes FGR. The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol 1988; 17: 201-207.
- 14. Hiatt W, Hoag S, Hamman R. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. Circulation 1995; 91: 1472-1479.
- 15. Meijer W, Hoes A, Rutgers D, et al. Peripheral arterial disease in the elderly. The Rotterdam Study. Arterioscler Thromb Vasc Biol 1998; 18: 185-192.
- 16. Stoffers H, Rinkens P, Kester A, et al. The prevalence of asymptomatic and unrecognised peripheral arterial occlusive disease. Int J Epidemiol 1996; 25: 282-290.
- 17. Fowler B, Jamrozik K, Norman P, Allen Y. Prevalence of peripheral arterial disease: persistence of excess risk in former smokers. Aust N Z J Pub Health 2002; 26: 219-224.
- 18. Criqui M, Langer R, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381-386.
- 19. Dormandy JA, Heeck L, Vig S. The fate of patients with critical leg ischaemia. Semin Vasc Surg 1999; 12: 142-147.
- 20. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 21. Rosenberg L, Palmer J, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. N Engl J Med 1990; 322: 213-217.
- 22. Fowkes G, Housley E, Riemersma R, et al. Smoking, lipids, glucose intolerance and blood pressure as risk factors for peripheral atherosclerosis compared with ischaemic heart disease in the Edinburgh Artery Study. Am J Epidemiol 1992; 135: 331-340.
- 23. Hobbs S, Bradbury A. Smoking cessation strategies in patients with peripheral artery disease: an evidence-based approach. Eur J Vasc Endovasc Surg 2003; 26: 341-347.
- 24. Shephard R, Balady G. Exercise as cardiovascular therapy. Circulation 1999; 99: 963-972.
- 25. Leng G, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev 2000; 2: CD000990.
- 26. Stewart A, Lamont P. Exercise for intermittent claudication. BMJ 2001; 323: 703-704.
- 27. Fowler B, Jamrozik K, Norman P, et al. Improving maximum walking distance in early peripheral arterial disease: randomised controlled trial. Aust J Physiother 2002; 48: 269-275.
- 28. Mohler E, Hiatt W, Creager M. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003; 108: 1481-1486.
- 29. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo–controlled trial. Lancet 2002; 360: 7-22.
- 30. Schedule of Pharmaceutical Benefits, February 2004. Canberra: Australian Government Department of Health and Ageing, 2004.
- 31. Donnelly R, Yeung J. Management of intermittent claudication: the importance of secondary prevention. Eur J Vasc Endovasc Surg 2002; 23: 100-107.
- 32. Chobanian A, Bakris G, Black H, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560-2572.
- 33. Lehto S, Niskanen L, Suhonen M, et al. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 1996; 16: 978-985.
- 34. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837-853.
- 35. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Antithrombotic Trialists’ Collaboration. BMJ 2002; 324: 71-86.
- 36. Goldhaber S, Manson J, Stampfer M, et al. Low-dose aspirin and subsequent peripheral arterial surgery in the Physicians’ Health Study. Lancet 1992; 340: 143-145.
- 37. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329-1339.
- 38. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342: 145-153.
- 39. Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J 2004; 25: 17-24.
- 40. Smith SC, Greenland P, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people. Role of traditional risk factors and non-invasive cardiovascular tests. Circulation 2001; 104: 1863-1867.
- 41. Fowkes F, Price J, Leng G. Targeting subclinical atherosclerosis. BMJ 1998; 316: 1764.
- 42. Leng G, Lee A, Fowkes F, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol 1996; 25: 1172-1181.
- 43. Mosca L. C-reactive protein — to screen or not to screen? N Engl J Med 2002; 347: 1615-1617.





Abstract
The prevalence of peripheral arterial disease (PAD) in people aged over 55 years is 10%–25% and increases with age; 70%–80% of affected individuals are asymptomatic; only a minority ever require revascularisation or amputation.
Patients with PAD alone have the same relative risk of death from cardiovascular causes as those with coronary or cerebrovascular disease, and are four times more likely to die within 10 years than patients without the disease.
The ankle–brachial pressure index (ABPI) is a simple, non-invasive bedside tool for diagnosing PAD — an ABPI less than 0.9 is considered diagnostic of PAD.
About half of patients with PAD (defined by an abnormal ABPI) have symptomatic coronary or cerebral vascular disease.
The ABPI is an independent predictor of coronary and cerebrovascular morbidity and mortality.
Patients with PAD require medical management to prevent future coronary and cerebral vascular events.
There are currently insufficient data to recommend routine population screening for asymptomatic PAD using the ABPI.