MDA National’s risk management committee reviewed the notifications and recognised that the potential costs of litigation required some intervention. The level of reported incidents paralleled the rapid uptake of the implant. The manufacturer modified the training program and released further information on the need for both doctor and patient to palpate the implant and document its presence after insertion (the manufacturer provided a sticker for doctor and patient to sign and put in the medical record). The problem of spiralling adverse incidents was reported in the medical press,5 but raising awareness alone was not sufficient to reduce the problem.
At the time of our initial review of the Implanon incidents, the High Court decision in the Cattanach v Melchior case for wrongful birth6,7 had not been handed down. The success of a claim for the cost of raising a healthy child meant that the potential cost of any unintended pregnancy claim could be high. While some states have changed legislation to prevent this type of claim, this is not yet national policy. Any claims lodged before these legislative changes will be heard in the light of the High Court decision.
MDA National’s response was in two stages: the initial response to contain the potential costs to the company, and the second to manage the clinical risk associated with the device.
MDA National undertook a further risk assessment using the Australian and New Zealand Standard for Risk Management (AS/NZS 4360: 1999).1 The goal was to develop a system to ensure that the procedure could be undertaken with minimal risk for the patient and reduced exposure to liability for the practitioner.
The Risk Management Standard defines the core principles of risk management (Box 1). It is a continuous process that establishes the context, identifies, analyses, evaluates, and treats the risk. The process is undertaken within a framework of ongoing communication, consultation, monitoring and review. The risk can be either accepted or treated in one of four ways: by reducing the likelihood, minimising the consequences, transferring in part or full, or avoiding.
At this point, MDA National approached the Royal Australian College of General Practitioners (RACGP) to assist with developing a consent form. The College identified that a consent process alone would not be sufficient, and developed a guideline that included a checklist for doctors and another for patients (Box 2).
The MDA National board accepted that adequate risk strategies could be developed to reduce the clinical risk of this treatment. It agreed to return the insertion of Implanon to the non-procedural general practice category, subject to compliance with established guidelines, from July 2004. In line with the risk-management process, this decision will remain under review.
The Standard for Risk Management (AS/NZS 4360: 1999) proved a useful tool to risk-assess and treat the problems that arose with the release of Implanon. It provides for ongoing review and monitoring of the proposed risk-management strategies. The Implanon story exemplifies an opportunity lost for risk assessment of a device in the clinical setting. A major intervention was required to halt the incidents. In future, a full risk assessment of the clinical implementation of a new product should be undertaken before its release. This would identify the risk barriers needed to minimise adverse incidents. If this had been undertaken, the one-year moratorium imposed on non-procedural general practitioners by MDA National might have been avoided.
The Royal Australasian College of Surgeons has established the Australian Safety and Efficacy Register of New Interventional Surgical Procedures (ASERNIP-S) to review new procedures and high-risk techniques.8 General practice has relied on licensing bodies, in this case the Therapeutic Goods Administration, to determine the safety and efficacy of drugs and devices. While this body approves products, it does not evaluate the potential risks in delivering the treatment. Ideally, manufacturers and medical indemnity insurers would refer new products, techniques and treatments identified as high-risk to the Royal Australian College of General Practitioners, which has the experience and skills to set standards and provide guidelines for general practitioners.9
1 Risk management overview from the Australian and New Zealand Standard1
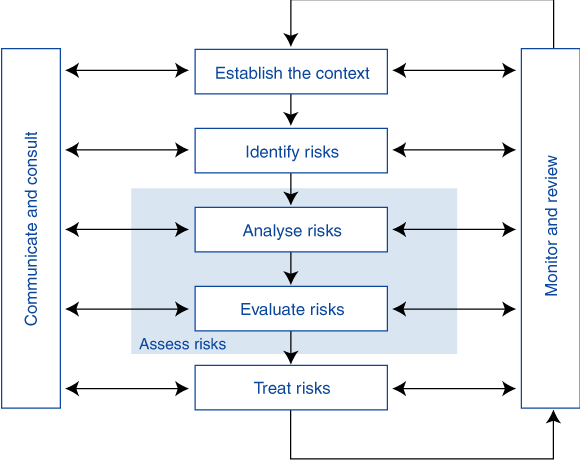
Reproduced from AS/NZS 4360:1999 (Figure 3.1)1 with permission of SAI Global Ltd. The Standard is available for purchase from www.sai-global.com and SAI Global Ltd, 286 Sussex Street, Sydney, NSW 2000.
2 Implanon checklist and consent form
The guideline developed by the Royal Australian College of General Practitioners covers three phases.
Initial consultation
The practitioner is required to:
Check for contraindications and allergies;
Plan the date of insertion; and
Disclose the risks.
Insertion
The practitioner is required to:
Verify the consent form, confirming the patient’s understanding of the risk of, and aspects particular to, the implant;
Check the device before insertion;
Palpate the implant after insertion;
Advise the patient of the recommended follow-up, including ensuring removal of the implant in 3 years; and
Complete all documentation.
The patient is required to:
Palpate the implant after insertion; and
Complete the consent form, acknowleging understanding of the treatment and that the implant is palpable.
This form becomes part of the patient’s medical record.
Removal
The practitioner is required to:
Document removal on the checklist and enter this in the medical record.
- 1. Standards Australia/Standards New Zealand. Australian/New Zealand Standard for Risk Management (AS/NZS 4360: 1999). Standards Australia, 1999.
- 2. Foran TM. New contraceptive choices across reproductive life, Med J Aust 2003; 178: 616-620. <MJA full text>
- 3. Cherry S. Implanon, the new alternative. Aust Fam Physician 2002; 31: 10.
- 4. Organon. Implanon Product information. Oss, Netherlands: NV Organon, 2001.
- 5. Munro J, Moss R. Implanon is effective but beware risks. Law in practice. Aust Doctor 2002; 10 May: 36.
- 6. Cattanach v Melchior [2003] HCA 38 (16 July 2003).
- 7. Gerber P. Failed sterilisations and the unwanted child: a new medicolegal minefield? Med J Aust 2004; 180: 123-125. <MJA full text>
- 8. Maddern GJ. The Australian Register of New Interventional Procedures — Surgical. RACS Bull 1999; 19: 49-53.
- 9. Standards for general practice. 2nd ed. Melbourne: Royal Australian College of General Practitioners, 2000.





Abstract
The contraceptive implant Implanon (Organon) was introduced in Australia in May 2001, and in the next 18 months was associated with an unprecedented number of adverse incident reports to medical indemnity insurers, including almost 100 unintended pregnancies.
The medical indemnity insurer, MDA National, responded to this by applying the Australian and New Zealand Standard for Risk Management (AS/NZS 4360: 1999) in two stages.
The first stage was to contain potential costs by moving the treatment into the general practice procedural category, resulting in a one-year moratorium on its use for most general practitioner members (prudential risk management).
The second stage was to manage the clinical risk by developing strategies to reduce identified risks associated with the procedure.
The Royal Australian College of General Practitioners (RACGP) was enlisted to develop guidelines for use of Implanon, with a consent form and checklists for doctors and patients, enabling MDA National to reinstate the treatment to the general practice non-procedural category.
This case demonstrates the need for early risk assessment and development of risk-management tools for new treatments and devices, a role that is appropriate for the RACGP.