While androgen deficiency may not shorten life expectancy, its pervasive effects on the development and maintenance of masculine sexual characteristics and anabolic status of somatic tissues can impoverish quality of life. Yet most of these effects are easily correctible by simple and cost-effective treatment.1 Appropriate management of androgen replacement therapy is rewarding for doctor and patient, whereas confused management can lead to mutual frustration.
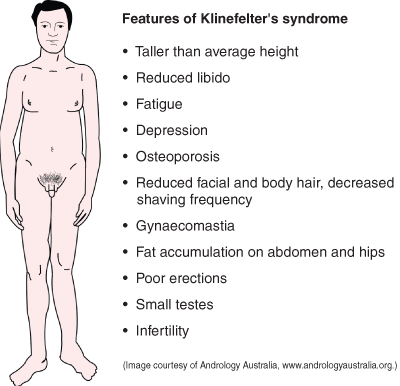
Common causes of androgen deficiency are shown in Box 1. Classical androgen deficiency is caused by testicular disorders that reduce testosterone production and by hypothalamic–pituitary disorders that reduce pituitary secretion of gonadotropins, the tropic drive to testosterone secretion. Common testicular causes are Klinefelter’s syndrome, which is associated with a 47,XXY karyotype, and developmental and toxic damage to the testes.
Pituitary tumours and their treatment are the most common cause of gonadotropin deficiency as part of hypopituitarism, while Kallmann’s syndrome is the most frequent form of isolated gonadotropin deficiency. An increasing number of genetic causes of this syndrome have been recognised, including inactivating mutations in the KAL1 gene (which encodes a cell-adhesion protein) and the FGFR1 (fibroblast growth factor receptor 1) gene. These interfere with the development or function of the gonadotropin-releasing hormone neurones. Kallmann’s syndrome often includes other abnormalities, such as anosmia and midline cranial defects, colour blindness, deafness and synkinesis. Inactivating mutations in genes such as the DAX-1 (adrenal hypoplasia congenita), GnRH (gonadotropin-releasing hormone) receptor and GPR54 (G protein-coupled receptor) genes cause isolated hypogonadotropic hypogonadism without the above clinical features.
Partial or transient androgen deficiency with mixed central and peripheral components may occur in chronic illness, severe acute illness, drug use, ageing and constitutional delay of puberty.
The prevalence of classical androgen deficiency is about 1 in 200 adult men. However, as recent evidence suggests that only 10%–30% of men with Klinefelter’s syndrome are diagnosed clinically during their lifetimes, 2,3 and as other causes of androgen deficiency have less distinctive clinical features, it is likely that they are underdiagnosed. However, age-related androgen deficiency is frequently overdiagnosed.
The clinical features of androgen deficiency depend on the epoch of life affected.1 Leydig cells in the testes secrete testosterone at adult male blood levels during fetal sexual differentiation, the perinatal androgen surge, and from puberty onwards.
During fetal life, androgen deficiency disrupts male sexual differentiation and somatic development. The effects are usually not correctible by postnatal hormone administration.
In adolescence, androgen deficiency manifests as late or incomplete sexual and somatic maturation. The most valuable clinical assessment is testicular examination, not only as testis growth is the first objective physical sign of puberty, but as serial observation of testis volume is the most reliable gauge of progression of normal puberty (see Box 2). For example, puberty has commenced when testis volume exceeds 4 mL (even before somatic manifestations are evident), and physical and mental virilisation are imminent when volume exceeds 8 mL. The other characteristic physical features of pubertal virilisation include growth of the larynx (voice change), height (bone growth spurt, epiphyseal fusion), musculature, genitals (enlarged phallus and scrotum with thickened, rugose, pigmented skin) and body hair (facial and truncal body hair, onset of shaving, subsequent temporal recession and balding).
In adult (post-pubertal) life, androgen deficiency causes regression of some features of virilisation. Symptoms are characteristically non-specific, notably reduced sense of well-being and energy levels (lethargy, easy fatigue, lack of stamina or endurance), reduced libido, mood changes (irritability, “short-fuse”, non-coping behaviour) and loss of physical, social and sexual drive. Flushing similar to the vascular instability in postmenopausal women occurs in some men with castrate testosterone levels, but is rare in less severe deficiency. Objective effects of androgen deficiency include mild to moderate (but not severe) reduction in haemoglobin level, weight gain and reduction in muscle and bone mass.
The effect of androgen on sexual function manifests primarily through effects on libido. However, the threshold is low, with libido maintained by low circulating testosterone levels.4,5 Sexual function is impaired only by severe androgen deficiency, and then characteristically through reduced libido, with the resulting reduced sexual activity rarely troubling the affected man. In men presenting with erectile dysfunction, the preserved libido indicates a low a priori likelihood of androgen deficiency. In such cases, empirical androgen therapy is inappropriate, as it may enhance libido but not erectile capacity.
Androgen deficiency is a clinical diagnosis confirmed by hormonal assays.1,6 Neither the clinical features, which are mostly non-specific, nor the hormonal findings, unless severe (ie, persistently castrate levels), are sufficient alone for diagnosis. Circulating testosterone level can be lowered by intercurrent medical conditions.
Clinical diagnosis depends on recognising clinical features in characteristic settings. Lifelong androgen replacement should not be recommended without a full history and careful physical examination, coupled with reliable hormonal assays.
The history should include:
childhood (cryptorchidism, hernia and hypospadias) and pubertal development (voice change, shaving onset and growth spurt relative to peers);
fertility history (paternity relative to opportunity);
known testicular abnormalities;
toxin exposure, medication and occupation; and
recent changes in sexual function and patterns of body hair.
Physical examination must include:
palpation of the testes for volume and consistency (atrophy);
assessment of the pattern of androgenisation (secondary sexual characteristics, especially body hair distribution, musculature and gynaecomastia); and
in men aged over 50 years, digital rectal examination to exclude palpable prostate pathology. This is essential, together with measurement of blood prostate-specific antigen level, before commencing androgen replacement therapy.
In adults, androgen deficiency may be considered in several clinical settings, notably infertility, sexual dysfunction, non-specific symptoms and self-diagnosis. Infertile men usually have isolated disorders of spermatogenesis (azoospermia or severe oligozoospermia, testicular atrophy), and only occasionally require androgen replacement when presenting with infertility. Nevertheless, as age-related decline in testicular function will be superimposed, these patients should have long-term follow-up. Men presenting with erectile or ejaculatory dysfunction are rarely androgen deficient.
A presumptive clinical diagnosis of androgen deficiency can be confirmed by measurement of blood levels of testosterone, sex hormone-binding globulin (SHBG), luteinising hormone (LH) and follicle-stimulating hormone (FSH) using well-calibrated immunoassays. Because of the diurnal rhythm in hormone secretion, blood samples should preferably be taken in the morning, when hormone levels are highest.6 Any abnormalities should be confirmed by testing on a second occasion. Laboratory features of different forms of androgen deficiency are shown in Box 3. In addition, bone density is a useful integrated measure of long-term prior androgen exposure and provides a baseline for monitoring the efficacy of androgen replacement therapy.
Clinical interpretation of the results of hormone assays relies on calibration to an appropriate reference population (men with normal testicular function), and currently most laboratories have not empirically verified their reference ranges. Andrology Australia, in conjunction with the Royal College of Pathologists of Australasia and the Australasian Association of Clinical Biochemists, now provides reference panels of samples for such standardisation.
Most circulating testosterone is protein-bound, and blood SHBG levels should be considered when interpreting extremes in blood testosterone concentration. However, derived testosterone measures, such as “free”, “bioavailable” and “calculated free” testosterone, and “free androgen (or testosterone) index”, which are increasingly reported, are not validated as diagnostic tests7 and have little practical diagnostic value. No valid, simple formula for “free” testosterone is available, and these measures add little to an accurate assay of total testosterone level combined with a thorough history and examination.
If androgen deficiency is confirmed, further tests to identify the underlying disorder are usually required, depending on the clinical features:
Primary (hypergonadotropic) hypogonadism: Small (< 4 mL) firm testes suggest Klinefelter’s syndrome or a Y chromosome microdeletion, which can be identified by karyotyping or genotyping, respectively (see case report, Box 4).
Secondary (hypogonadotropic) hypogonadism:
In delayed puberty, simple chronological constitutional delay must be differentiated from permanent hypogonadotropic hypogonadism and the effects of underlying chronic illness.
In delayed puberty or infertility, anosmia, midline cranial defects, synkinesis or family history suggest Kallmann’s syndrome or another form of idiopathic hypogonadotropic hypogonadism. Genotyping to detect a mutation (eg, in the KAL1, FGRF1, DAX-1, GPR54 or GnRH-receptor genes) may be required.
Headache or visual disturbance suggests a pituitary tumour, which should be investigated with measurement of serum prolactin (prolactinoma), serum thyroxine, thyroid-stimulating hormone, cortisol and insulin-like growth factor (hypopituitarism), and, if indicated, magnetic resonance imaging of the pituitary.
Hepatic cirrhosis, heart failure, diabetes mellitus, pigmentation or relevant family history suggest haemochromatosis, which requires iron studies and genotyping.
Tertiary hypogonadism (androgen resistance or insensitivity): Sequencing of the androgen receptor or coregulator gene may be required to detect a mutation as a basis for genetic counselling.
Androgen replacement therapy (ART) should be started only after a definite diagnosis is established, and contraindications (prostate or breast cancer) have been excluded. For older men (over 50 years), untreated prostate disease should be excluded by digital rectal examination and measurement of blood PSA level.
Guidelines for androgen prescribing are shown in Box 5.
Classical androgen deficiency: ART is required and should continue lifelong with age-appropriate monitoring. Any underlying pituitary or testicular disorder should also be treated appropriately.
Non-classical forms of androgen deficiency: ART is rarely justified. Acute androgen deficiency caused by catabolic states, such as critical illness, burns, severe trauma or major surgery, is usually rectified by resolution of the precipitating condition. Similarly, androgen deficiency associated with chronic disease or drug use does not necessarily require treatment, especially if the underlying disorder can be alleviated (eg, depression, obesity and alcohol misuse). However, ART may be warranted if the androgen deficit is severe or prolonged, although there is no clear evidence of sustained objective benefits in chronic systemic illness, obesity or depression. Use of ART for age-related androgen deficiency is currently controversial, with no evidence of objective benefit.
Short-term “trials” of ART are undesirable, as they only prolong diagnostic uncertainty and, once ART is begun, it is difficult, time-consuming and frustrating for both doctor and patient to evaluate the need for ongoing therapy. Transient responses (including placebo responses) are likely, and months of withdrawal from ART (with accompanying deficiency symptoms) may be needed before the gonadal axis can be properly investigated. Therefore, it is essential to make a clear decision on the need for lifelong testosterone treatment, with the option of later review if the decision is not to start it.
Testosterone products available in Australia and their modes of delivery are shown in Box 6.
Injectable testosterone is the long-time standard treatment and is most cost-effective. It is applicable in almost all clinical situations, except when bleeding disorders preclude deep intramuscular injection. Injectable products comprise testosterone esters or testosterone enanthate in an oil vehicle, which releases testosterone gradually. Weekly injections are desirable to approximate steady-state delivery, but injections every two weeks are the usual compromise. Longer spacing leads to fluctuating blood levels and unpleasant deficiency symptoms.
Transdermal testosterone patches are a suitable alternative to injectable testosterone. Transdermal therapy requires the delivery of milligrams of testosterone daily, straining the limits of this technology, which was originally designed to deliver only micrograms. Patches therefore require absorption enhancers, which commonly cause skin irritation, but, if tolerated, can be a very satisfactory long-term option.8 Other transdermal delivery systems based on creams, gels and sprays are less irritating and more cosmetically acceptable but more expensive;9 none are registered in Australia.
Testosterone undecanoate is the only safe oral androgen, being free of the hepatotoxicity of synthetic 17-α alkylated androgens. However, it is several times more expensive, has low and erratic bioavailability and can cause gastrointestinal intolerance. Consequently, it is a second-line agent useful when parenteral testosterone administration is unacceptable (eg, in bleeding disorders) or when rapid discontinuation of treatment may be an advantage (eg, in older men if prostate cancer is diagnosed).
Testosterone implants are inserted subdermally via a small skin incision in the lateral abdominal wall or buttocks using a trochar and cannula under local anaesthesia.10 A single implantation of 800 mg testosterone (four 200 mg pellets) is usually adequate for 6 months.11 The major drawbacks are the need for skill in minor office-type surgery for administration, and the risk of extrusion (in about 10% of implantation procedures, usually of a single pellet). Implants are also not desirable in older men who may need to cease ART rapidly. Implants should be replaced only after the return of androgen-deficiency symptoms and confirmation of low blood testosterone concentration (< 15 nmol/L).
Unregistered products that deliver testosterone and other androgens are increasingly available. In the United States, dehydroepiandrosterone (DHEA), its sulfate (DHEAS), androstenedione and other androgenic steroid precursors were reclassified in the 1990s as foods rather than drugs, allowing them to be sold over the counter and avoiding surveillance by the US Food and Drug Administration. In Australia, these drugs are prohibited and cannot be legally imported without a permit. Illegal purchases of DHEA via the Internet are responsible for most seizures of androgens by the Australian Customs Service. The extremely limited legitimate market for DHEA (women with total adrenal failure) is allowed for under the Special Access Scheme. The other agents have no proven role in therapeutics.
Under extemporaneous preparation provisions of state pharmacist registration, licensed pharmacists may provide preparations of testosterone and other steroid precursors for a named patient as directed by a valid medical prescription. This is commonly in the form of a troche, a medicated lozenge that allows transbuccal absorption, bypassing the hepatic first-pass metabolism after oral ingestion. However, transbuccal absorption is poor and often inconsistent, and these non-proprietary troches have not been evaluated for pharmacological and clinical efficacy. A commercial oral lozenge has recently been marketed in the United States after substantial technological improvement but is not registered for marketing in Australia.
Monitoring of ART aims to maintain consistent androgen replacement levels and to detect side effects, which are rare and include polycythaemia, sleep apnoea, pain at the injection site, implant extrusion and patch-induced skin irritation. Measurement of blood testosterone levels is of no value in men taking oral testosterone because of the unpredictable pharmacokinetics. If problems occur in those using steady-state delivery systems (transdermal delivery, implant, and, to a lesser extent, injection), then measurement of trough blood levels (ie, immediately before a dose is due) may be useful. Bone density should be measured at 1–2-yearly intervals to verify restoration towards age-specific norms.
Testosterone usually relieves the symptoms of classical androgen deficiency rapidly (over days to weeks). Inadequate replacement is most commonly caused by an inadequate regimen (eg, monthly injections) or poor compliance (usually because of dislike of the mode of administration), rather than suboptimal tailoring of the regimen to the individual. Symptoms that persist despite the standard dose of injectable testosterone over some weeks are unlikely to be caused by androgen deficiency.
Men with classical androgen deficiency have reduced prostate volume and blood prostate-specific antigen (PSA) levels compared with their age peers.12,13 As it is plausible that androgen deficiency partially protects against prostate disease, and that restoring androgen exposure increases risk to that of eugonadal men of the same age, 14 men using ART should have age-appropriate surveillance for prostate disease. This should comprise digital rectal examination and blood PSA measurement at regular intervals determined by age and family history.
The clinical diagnosis of androgen deficiency is more difficult in older men. There is consensus that, in healthy men, ageing is accompanied by a gradual, modest and variable decline in blood testosterone levels, which is accelerated in the presence of chronic disease.15 The non-specific clinical features of ageing are similar to those of androgen deficiency. This has led to the suggestion that ageing is a reversible form of androgen deficiency. However, this is not supported by clinical evidence. More than 10 well-designed, randomised, placebo-controlled clinical trials of the effects of testosterone or other androgens in older men have consistently shown only modest changes in body composition (1–3 kg increased muscle and reduced fat mass), and no objective or reproducible benefits in bone, muscle, quality of life or psychosexual function.16 While trials with more defined target subpopulations of older men and more refined objectives may identify significant benefits, so far there is no basis for androgen treatment of older men, unless they have overt androgen deficiency17 (see case report, Box 7).
Nevertheless, the prospect of rejuvenation by androgen therapy has great appeal to an affluent, ageing population, making it ideal for effective mass marketing. Over the last decade, global testosterone sales have increased sharply, almost exclusively because of increased sales in the United States. Contributing factors may be sophisticated multi-media marketing directed at the general public, and the spread of franchised impotence and “andropause” clinics. The problem of androgen misuse requires active professional education, such as that being pioneered by Andrology Australia, the Australian Centre of Excellence in Male Reproductive Health (www.andrologyaustralia.org).
1: Common causes of androgen deficiency
Classical androgen deficiency
Testicular disorders
Klinefelter’s syndrome and variants (mosaic)
Cryptorchidism and defects of testis development
Orchitis
Orchidectomy (advanced prostate cancer, bilateral testicular cancer)
Toxin exposure (cancer chemotherapy or radiotherapy, environmental, occupational and domestic toxins)
Hypothalamic–pituitary dysregulation
Idiopathic hypogonadotropic hypogonadism and variants:
Kallmann’s syndrome (associated with anosmia and midline cranial defects; caused by mutations in the KAL1 or FGRF1 [fibroblast growth factor receptor 1] genes)
Other genetic causes (including inactivating mutations in the DAX-1 [adrenal hypoplasia congenita], GnRH-receptor or GPR54 [G protein-coupled receptor] genes)
Pituitary tumour and treatment (surgery, irradiation)
Haemochromatosis
Craniopharyngioma
Partial or transient androgen deficiency
Constitutional delay of puberty
Acute critical illness, burns, major trauma or surgery
Drug use (eg, opiates, glucocorticoids, anabolic steroids)
Chronic disease and its treatment
Ageing (“late-onset” androgen deficiency)
GnRH = gonadotropin-releasing hormone.
2: Measurement of testicular volume
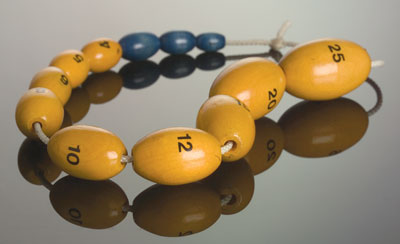
An orchidometer is the standard instrument for measuring the testes. The beads are marked with the corresponding volume (mL). (Photograph courtesy of Danielle Edwards, Austin Health, Melbourne, VIC.)
3: Laboratory features of different forms of androgen deficiency
Condition |
Functional status |
Testosterone |
LH and FSH |
||||||||
Primary (hypergonadotropic) hypogonadism |
Testicular lesion with hypergonadotropic state due to negative feedback |
Low |
High |
||||||||
Secondary (hypogonadotropic) hypogonadism |
Hypothalamopituitary defect in LH and FSH secretion leading to reduced testosterone secretion |
Low |
Low or normal |
||||||||
Tertiary hypogonadism (androgen resistance or insensitivity) |
End-organ resistance due to androgen-receptor mutation or dysfunction |
High |
High |
||||||||
Delayed puberty |
Spectrum from transient to permanent hypogonadotropic hypogonadism |
Low |
Low or normal |
||||||||
Acute catabolic states (critical illness, burns, severe trauma, major surgery) |
Acute, transient variant of hypogonadotropic hypogonadism |
Low |
Low |
||||||||
Chronic systemic illness, obesity, severe weight loss |
Mild chronic variant with a mixture of primary and secondary (mixed) hypogonadism |
Low or low–normal |
Normal |
||||||||
Ageing |
Mild chronic variant of mixed hypogonadism |
Low or low–normal |
Normal or high |
||||||||
LH = luteinising hormone. FSH = follicle-stimulating hormone. |
|||||||||||
4: Case report — a young man with low libido
Presentation: A 25-year-old man presented with low libido compared with his friends. He was tall and slender, but physical examination otherwise showed no abnormalities apart from a minor degree of gynaecomastia and small, firm, pea-like testicles (about 2 mL volume).
What is the likely diagnosis?
Klinefelter’s syndrome.
What tests should be performed?
Measurement of serum levels of total testosterone (typically very low), luteinising hormone (typically high) and follicle-stimulating hormone (typically high), and chromosomal analysis (XXY, or rarely mosaic or XX).
What is the management?
Lifelong testosterone replacement. Consider need for counselling and breast reduction surgery if gynaecomastia persists when receiving maintenance testosterone replacement.
Can the patient be fertile?
No, not through natural intercourse, except in very rare cases of mosaicism. Fertility is possible with testicular sperm extraction and intracytoplasmic sperm injection.
5: Guidelines for androgen prescribing*
A. Men with classical androgen deficiency due to hypothalamic–pituitary or testicular disorders
PBS authority requirement: Androgen deficiency in males with established pituitary or testicular disorders.
Comment: No restriction on prescribing for men with classical androgen deficiency.
B. Men without classical androgen deficiency
PBS authority requirement: Androgen deficiency in males 40 years and older who do not have established pituitary or testicular disorders other than ageing, confirmed by at least 2 morning blood samples taken on different mornings. Androgen deficiency is confirmed by testosterone level < 8 nmol/L, or 8–15 nmol/L with high luteinising hormone level (> 1.5 times the upper limit of the eugonadal reference range for young men).
Comment: Disallows PBS subsidy for prescribing to older men who do not have classical androgen deficiency but may have age-related partial, mild or biochemical androgen deficiency, unless severe. Among older men, clinical trial data provide no evidence of objective benefit from testosterone treatment. Therapy directed at the underlying cause of lowered testosterone, such as lifestyle, obesity and chronic illness, may be effective.
C. Boys with micropenis or delayed puberty
PBS authority requirement: Micropenis, pubertal induction, or constitutional delay of growth or puberty, in males under 18 years of age.
Comment: No restriction on prescribing; should be managed by an experienced paediatric endocrinologist.
PBS = Pharmaceutical Benefits Scheme.
* These guidelines were developed by the Endocrine Society of Australia in 20006 and adopted by the Pharmaceutical Benefits Scheme for national subsidised prescribing. They continue to reflect current knowledge, but may need to be revised when new evidence becomes available.
6: Testosterone products in Australia
Product |
Dose |
Comments |
|||||||||
Injection (deep intramuscular) Testosterone esters or enanthate (250 mg) in oil vehicle |
250 mg every 2 weeks |
Advantages: Widely available; cheapest; suits most clinical situations |
|||||||||
Transdermal Testosterone patch (2.5 mg or 5.0 mg), Cream (5%) or Gel (1%) |
5.0 mg daily (may start with 2.5 mg daily; may titrate up to 7.5 mg) |
Advantages: Suitable in bleeding disorders |
|||||||||
Subdermal implant Testosterone pellet (100 mg or 200 mg) |
800 mg 6-monthly |
Advantages: Convenience — infrequent administration suits younger men |
|||||||||
Oral Testosterone undecanoate (40 mg) in oil-filled capsule |
160–240 mg in 2–3 divided doses daily |
Advantages: Only safe oral androgen; suitable in bleeding disorders |
|||||||||
Non-proprietary forms Troche Dragee or Ointment |
Ad hoc dosage |
Formulated by compounding pharmacist on a prescription for a named individual; sale allowed under extemporaneous preparation provisions of State pharmacist registration |
|||||||||
7: Case report — androgen deficiency and ageing
Presentation: A 50-year-old man presented with lethargy, difficulty in concentrating, depression and erectile dysfunction. Physical examination showed no abnormalities and was consistent with his age. Serum testosterone level was 9 nmol/L (reference range, 7–20 nmol/L), while serum levels of luteinising hormone and follicle-stimulating hormone were reported as “normal”.
Would this patient benefit from testosterone therapy?
No. There is currently no good evidence that the symptoms would be consistently alleviated, or that the risks of bone fracture, dementia or cardiovascular disease would be reduced by this treatment.
History: The patient had undergone a 3-month trial of testosterone injection a year previously and had an excellent response in the first month. He stopped at 3 months because the injections no longer seemed to be working.
Should testosterone be restarted? At a higher dose?
No. This short-term response is classically a placebo effect. There is no justification for higher doses and they will not be any more effective.
- David J Handelsman1
- Jeffrey D Zajac2
- 1 ANZAC Research Institute, Concord Hospital, Sydney, NSW.
- 2 Department of Medicine, University of Melbourne, Austin Hospital, Melbourne, VIC.
D J H: Consultant to Besins (France) and Lawley (Australia) for clinical trials of testosterone cream and gel. J Z: None identified..
- 1. Handelsman DJ. Androgen action and pharmacologic uses. In: DeGroot LJ, editor. Endocrinology. 4th ed. Philadelphia: W B Saunders, 2001: 2232-2242.
- 2. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003; 88: 622-626.
- 3. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn 1997; 17: 363-368.
- 4. Gooren LJ. Androgen levels and sex functions in testosterone-treated hypogonadal men. Arch Sexual Behaviour 1987; 16: 463-473.
- 5. Buena F, Peterson MA, Swerdloff RS, et al. Sexual function does not change when serum testosterone levels are pharmacologically varied within the normal male range. Fertil Steril 1993; 59: 1118-1123.
- 6. Conway AJ, Handelsman DJ, Lording DW, et al, on behalf of the Endocrine Society of Australia. Use, misuse and abuse of androgens: the Endocrine Society of Australia consensus guidelines for androgen prescribing. Med J Aust 2000; 172: 220-224. <MJA full text>
- 7. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. End Rev 2003; 24: 313-340.
- 8. Arver S, Dobs AS, Meikle AW, et al. Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men. Clin Endocrinol (Oxf) 1997; 47: 727-737.
- 9. Wang C, Swedloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab 2000; 85: 2839-2853.
- 10. Handelsman DJ. Testosterone implants: a manual of scientific and clinical information. Auckland: Stredder Print, 1991.
- 11. Handelsman DJ, Conway AJ, Boylan LM. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 1990; 71: 216-222.
- 12. Jin B, Conway AJ, Handelsman DJ. Effects of androgen deficiency and replacement on prostate zonal volumes. Clin Endocrinol (Oxf) 2001; 54: 437-445.
- 13. Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 2003; 88: 2049-2054.
- 14. Handelsman DJ. The safety of androgens: prostate and cardiovascular disease. In: Wang C, editor. Male reproductive function. Boston: Kluwer Academic Publishers, 1998: 173-190.
- 15. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87: 589-598.
- 16. Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc 2003; 51: 101-115.
- 17. Barrett-Connor E, Bhasin S. Time for (more research on) testosterone. J Clin Endocrinol Metab 2004; 89: 501-502.





Abstract
Androgen deficiency is a clinical diagnosis confirmed by hormone assays.
Among younger men, androgen deficiency is usually due to underlying hypothalamopituitary or testicular disorders.
Androgen replacement therapy should be started after proof of androgen deficiency and should continue lifelong with monitoring.
Men presenting with erectile dysfunction should be evaluated for androgen deficiency, but it is an uncommon cause; if overt androgen deficiency is confirmed, an underlying disorder needs further specialist investigation.
In the absence of characteristic underlying testicular or pituitary disorders, new diagnosis of androgen deficiency in older men is difficult because of the non-specific symptoms and the decline in blood testosterone levels seen in healthy ageing and chronic medical disorders.
There remains no convincing evidence that androgen therapy is either effective treatment or safe for older men unless they have frank androgen deficiency.