Obesity is the excess accumulation of body fat. It can be assessed by the body mass index (weight in kilograms divided by the square of height in metres [BMI]). The relationship of BMI to total body and visceral fat, and consequent complications, varies between ethnic groups1 (Box 1). Asian populations, particularly those from South East Asia and the Indian subcontinent, have more fat and more comorbidities for any given BMI, resulting in different suggested BMI cut-off points.3 In contrast, Polynesians have higher BMI cut-off points.4
The risk of comorbidities rises with increasing BMI, with a mild rise in the overweight range, moderate in class I, severe in class II and very severe in class III obesity. As well as the total fat, distribution is important to the incidence of comorbidities. Central abdominal fat, particularly visceral fat, is a risk factor for the metabolic syndrome. Waist circumference cut-off points in white European populations are shown in Box 2.
Adult BMI cut-off points cannot be used for assessing children and adolescents, as BMI varies throughout childhood, being high in the 2nd year of life, dropping to a nadir at age 4–7 years and then rising again to adult values (Box 3). BMI-for-age charts, such as those developed by the Centers for Disease Control and Prevention in the United States,6 can be used in clinical practice to assess and monitor BMI over time in children (Box 3). Overweight is defined as BMI between the 85th and 95th percentiles, and obesity as BMI > 95th percentile,7 but these definitions are arbitrary, as, unlike adult BMI cut-offs, they are not linked to morbidity data. Although a table of age- and sex-specific cut-offs developed for epidemiological research8 allows international comparison of trends in overweight and obesity in children, it is not intended for routine clinical use.
In children and adolescents, as in adults, waist circumference is strongly correlated with abdominal fat and markers for comorbidities, such as adverse lipid and glucose profiles and hypertension.9 However, there are no internationally accepted criteria for waist circumference in this age group and, as for adults, racial and ethnic variations exist. For example, African American, Mexican American and Mohawk Indian children carry more abdominal fat than white children.10
The prevalence of obesity appears to have more than doubled in Australian adults in the decades from 1980 to 2000. A 1980 survey of people aged 25–64 years living in capital cities found that 7.1% were obese.11 In contrast, the 1999–2000 Australian Diabetes, Obesity and Lifestyle (AusDiab) study found in the same urban age group that 18.4% were obese, a 2.5-fold increase.12 Overall, the AusDiab study found, among 11 000 adults from around Australia, that 48.2% of men and 29.9% of women were overweight, while 19.3% of men and 22.2% of women were obese.12
The aetiology of obesity is complex, with both environmental and genetic influences. The recent increase in prevalence is clearly due to the continuous availability of high-energy foods, together with a major reduction in the obligatory need for physical activity that has characterised human existence until very recently.
However, not everyone in an obesogenic environment becomes obese, indicating that a genetic predisposition is required. Studies of twin pairs reared together or apart suggest that about 70% of the influence on body weight is genetic, while about 30% is environmental.14 The dominance of genetic influences has been confirmed by adoption studies, which found that adoptees resemble their biological parents in body size, with very little resemblance to their adoptive parents.15 Some genes that could predispose to obesity have been identified (including genes encoding leptin, the leptin receptor, pro-opiomelanocortin and the melanocortin-4 receptor), but many more are probably as yet undiscovered.16
In children, environmental contributors to obesity include prolonged television viewing, playing of computer and video games,17 decreased physical activity (especially incidental activity) and increased consumption of energy-dense foods and sugar-containing drinks.18
Obesity has been linked to complications in many body systems (Box 4).
A weight loss and maintenance strategy for individual patients is outlined in Box 5. The weight-loss phase involves establishing a negative energy balance by reducing energy intake and increasing physical activity. Most motivated patients can succeed in this phase, but different strategies suit different individuals.
The next phase, weight maintenance, is more difficult and requires more emphasis. Lifestyle modification plus pharmacotherapy, if required, offer the best chance of success. For those who have failed in weight maintenance, bariatric surgery currently produces the best results and should be considered.
Clearly, it is important to set realistic goals from the start. Weight loss that is useful from a medical point of view (5%–10%) often does not satisfy patients. It may help to point out that more modest losses may be easier to maintain in the long term.
It is important to ensure that patients are ready and enthusiastic about attempting weight loss, as their cooperation is essential. A negative energy balance must be established, by reducing energy intake and increasing energy expenditure. A practical way to reduce energy intake is to limit fat intake. Patients may need information about foods that contain fat. It may help to also limit carbohydrates, especially those with a high glycaemic index (rapidly digested and absorbed carbohydrates), as high insulin levels can encourage weight gain22,23 (see also National Health and Medical Research guidelines on managing obesity,7 section 5, available at www.obesityguidelines.gov.au/pdf/adults_part5.pdf). A reduction of 2500 kJ (600 calories) from the stable prior intake is generally advised, which should lead to weight loss of 0.6 kg per week. This can be calculated from a diet diary kept over a week before starting treatment. The advice of a trained dietitian will be of great help.
Increased physical activity is an important component of lifestyle modification. The increase must be substantial (80 minutes of moderate-intensity activity per day), but cannot usually be achieved immediately. A more modest initial target can be set (eg, 30 minutes of walking 3–5 days per week) and increased gradually. While reduced energy intake is the major method of losing weight, it has been shown that a high level of physical activity is essential to assist with maintaining weight loss. Irrespective of its impact on weight, physical activity has wider benefits on well-being, including improved cardiovascular fitness.
A Cochrane review on the effectiveness of intervention by health professionals in weight management concluded that “there are few solid leads about improving obesity management, although reminder systems, brief training interventions, shared care, in-patient care and dietitian-led treatments may all be worth further investigation”.24
For children and adolescents, the broad principles of management are well recognised and comprise behaviour modification (eg, patterns of television watching, computer use, socialising and leisure activity), family support, dietary change, increased physical activity, decreased sedentary behaviour, and a developmentally appropriate approach.
To be effective, therapy must take account of family influences on food and activity habits.24 Parental involvement is necessary with both young children and adolescents. Several studies have now shown that long-term (2–10-year) maintenance of weight loss can be achieved with family-based intervention.25 There is no direct evidence on which dietary modification is most effective in this age group. Interventions should follow national nutrition guidelines and emphasise lower-fat options, increasing vegetable and fruit intake, healthier snack-food choices and probably decreased portion sizes. Reducing soft drink and fruit juice intake is also important.18
Participation in an exercise program is a long-term predictor of successful weight control in children.26 The type of exercise appears important for sustained weight loss. While both “lifestyle” (eg, walking and cycling) and programmed aerobic exercise promote weight loss in the initial phase, “lifestyle” exercise is more likely to be continued long term.
In pre-adolescents, an approach using parents as the exclusive agents of lifestyle change appears superior to a child-centred approach, where the child is expected to take major responsibility for making changes.27,28 Thus, sessions involving one or both parents without the child are likely to be the most effective.
In adolescents, features of successful interventions include separate sessions for the adolescent and parent, and a structured but flexible program that encourages sustainable modifications in lifestyle, relationships and attitudes29 (see case report, Box 6). There is also a report of success, at least in the short term (3 months), with a 4-month behavioural weight control program for overweight adolescents initiated in a primary care setting and extended through telephone and mail contact.30
The National Health and Medical Research Council recently published clinical practice guidelines for management of overweight and obesity in children and adolescents, which review in detail the evidence on interventions.31
The use of drugs to assist in management of obesity has always been controversial. This is partly due to the widespread belief that losing weight and maintaining weight loss is simply a matter of exercising free will, which has even led to a suggestion that it is unethical to be obese.32 However, this view cannot explain why the overwhelming majority of obese individuals regain weight after successful weight loss,33 despite an often desperate desire to remain lean. The discovery of the hormone leptin in 1994 shed light on this paradox.
Leptin is a cytokine hormone produced in fat cells in proportion to their size. It is secreted into the bloodstream and crosses the blood–brain barrier via an active, saturable process. It acts in the hypothalamus to alter the expression of neurotransmitters (eg, it inhibits production of neuropeptide Y and stimulates production of melanocyte-stimulating hormone), resulting in suppression of hunger and an increase in spontaneous activity. Hence, as weight increases, fat cells increase in number and size, increasing the production of leptin, which feeds back to inhibit food intake and increase energy expenditure, limiting weight gain. It appears that a genetically lean individual will gain an extra 7–8 kg before leptin increases sufficiently to stop the weight gain. Individuals who gain more than this amount must be unresponsive to the hormone, either because it cannot enter the brain efficiently or because there is a mutation in one of the many steps required for leptin action, such as at the melanocortin receptor. When obese individuals with a high leptin level lose weight, the leptin level falls dramatically,34 resulting in relative leptin deficiency.
It is known from a study of two children with a homozygous mutation in the leptin gene that leptin deficiency leads to insatiable hunger.35 Thus, it is likely that weight-reduced obese individuals regain weight despite a great desire to remain lean because they cannot tolerate hunger long term in the face of abundant food. This has led to reappraisal of the role of drugs and surgery in management of obesity (see case report, Box 7).
Drugs available for treating obesity are shown in Box 8. Both the intestinal lipase inhibitor orlistat and the serotonin- and noradrenaline-reuptake inhibitor sibutramine have been shown to limit weight regain in large randomised placebo-controlled trials.37,38 The lack of long-term studies of the noradrenergic agonists phentermine and diethylpropion limits their usefulness in long-term management.
Some antidepressants affect body weight, including the selective serotonin-reuptake inhibitors (fluoxetine, paroxetine, fluvoxamine, citalopram and sertraline) and the serotonin- and noradrenaline-reuptake inhibitor venlafaxine. The effects of fluoxetine are best studied, with several studies showing a modest but reproducible effect on weight loss. These drugs are not approved for treating obesity, but should be the drugs of choice when treating depression in overweight patients.
Many compounds are currently undergoing clinical trials including:
leptin and leptin analogues;
topiramate, an antiepileptic drug with appetite-suppressant action;
rimonabant, an inhibitor of the cannabinoid-1 receptor;
amylin, a protein secreted by pancreatic beta cells; and
AOD 9604, a fragment of the growth hormone molecule.
Many other compounds are in earlier stages of development.
Surgery should be considered for patients with significant comorbidities associated with obesity, especially if medical therapy has failed repeatedly. Studies in Australia39 and overseas40 have shown major benefits of gastric surgery on weight loss, diabetes, hypertension, dyslipidaemia, insulin sensitivity, sleep apnoea, asthma, infertility and quality of life. For example, a study of 50 patients with type 2 diabetes and an initial mean BMI of 48 kg/m2 found that, a year after laparoscopic gastric banding, fasting serum glucose level had decreased from 9.4 to 6.2 mmol/L, glycosylated haemoglobin level from 7.8% to 6.2%, serum triglycerides from 2.4 to 1.4 mmol/L, and blood pressure from 154/96 to 130/79 mmHg, while high-density lipoprotein cholesterol level had increased from 1.03 to 1.22 mmol/L.41 Of the 29 patients who had been taking oral hypoglycaemic agents, only eight still required these, after an average 27 kg weight loss. While laparoscopic gastric banding (Box 9) is becoming the favoured approach because of its reversibility and low morbidity, some surgeons still perform gastric stapling or biliopancreatic diversion with good outcomes.42 Bilio-pancreatic diversion is indicated when previous restrictive surgery has failed and could be considered in superobesity.
1: Definitions of obesity in adults2
|
Body mass index (kg/m2) |
||||||||||
Classification |
White European |
Asian |
Polynesian |
||||||||
Underweight |
< 18.5 |
< 18.5 |
|
||||||||
Normal range |
18.5–24.9 |
18.5–22.9 |
|
||||||||
Overweight |
25.0–29.9 |
23.0–25.9 |
26.0–31.9 |
||||||||
Obese |
> 30.0 |
> 26.0 |
> 32.0 |
||||||||
Class I |
30.0–34.9 |
26.0–29.9 |
|
||||||||
Class II |
35.0–39.9 |
30.0–35.0 |
|
||||||||
Class III |
> 40.0 |
> 35.0 |
|
||||||||
2: Waist circumference associated with increased risk of metabolic complications in white European adults*
|
Increased risk |
Substantially increased risk |
|||||||||
Men |
≥ 94 cm |
≥ 102 cm |
|||||||||
Women |
≥ 80 cm |
≥ 88 cm |
|||||||||
* Reported by the World Health Organization,2 based on a study of 4881 adults in the Netherlands.5 Note that waist circumference cut-offs are population-specific. |
|||||||||||
3: Example of chart of body mass index (BMI) for age6
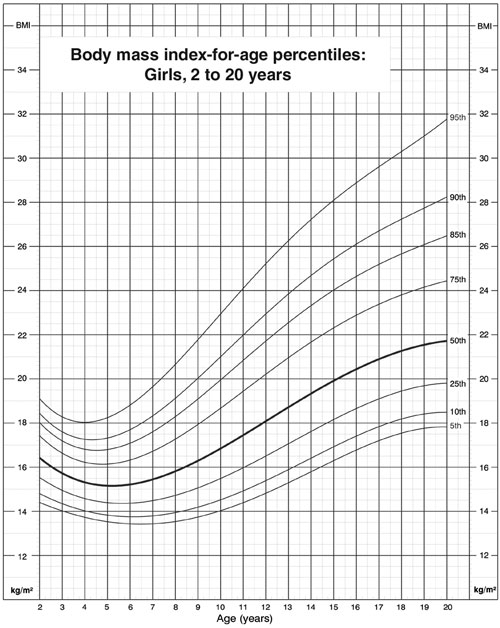
Charts available from Centers for Disease Control and Prevention, National Center for Health Statistics www.cdc.gov/growthcharts
4: Complications of obesity
Cardiovascular: Hypertension, dyslipidaemia, increased risk of coronary heart disease and stroke
Respiratory: Obstructive sleep apnoea, asthma
Endocrine: Glucose intolerance, insulin resistance, type 2 diabetes, polycystic ovary syndrome
Orthopaedic: Back pain, osteoarthritis, flat feet
Dermatological: Acanthosis nigricans, skin tags, intertrigo
Gastrointestinal: Non-alcoholic steatohepatitis, reflux oesophagitis, gallstones
Psychosocial: Social isolation and discrimination, decreased self-esteem, binge-eating disorder, bulimia, and depression
Other: Increased risk of breast and other cancers, increased intracranial pressure, proteinuria
5: Management of obesity in motivated adult patients
Step 1. History, examination and investigation (plus management of comorbidities if not resolved by weight loss)
Step 2. Weight loss (3–12 months)
Lifestyle modification (decrease energy intake and increase physical activity)
Diet
Low-fat, reduced-carbohydrate diet
Very low energy diet (VLED) (commercial, over-the-counter mixture of essential nutrients with defined energy content, usually supplied as a powder to be mixed with water and drunk three times daily)
Meal replacement program (eg, commercial program that provides preprepared meals)
Commercial weight loss centre
Step 3: Weight maintenance (lifelong)
Lifestyle modification
Pharmacotherapy (if required)
Step 4: Bariatric surgery (for obese patients who have failed medical therapy)
6: Case report — an overweight adolescent
Presentation: A 13-year-old girl presented to her general practitioner with a respiratory tract infection. During the consultation, her mother commented that she was concerned about her weight and was being teased about this at school. She had left her previous school because of bullying. Aware of the time needed to discuss this issue, the GP made a separate appointment for the girl and her mother to return.
History: The girl was an only child and appeared to have good relationships with her parents and peers. Her general health was good. Several family members were obese (mother, maternal aunts, three grandparents), and the mother described the family as “heavy-boned”. However, there was no significant family history of other disorders associated with insulin resistance (eg, type 2 diabetes, premature heart disease). The girl’s lifestyle was sedentary, with her main interests being playing music, sewing, reading and talking on the telephone. She was driven to and from school and watched about 3 hours of television per day. Her diet included at-risk features, such as occasionally skipping breakfast, consuming full cream milk, “something nice” for morning and afternoon tea and about 500 mL of soft drink per day, regularly buying food from a milk-bar in the afternoon, and “grazing” at home (eg, on biscuits from the cupboard).
Examination: Weight was 72.6 kg (> 97th percentile) and height 161.5 cm (< 75th percentile), giving a body mass index (BMI) of 28.0 kg/m2 (> 95th percentile for age; adult overweight range). Her waist circumference was 85 cm (adult “at risk of metabolic complications” range). She was in mid-puberty, with no striae on the abdomen or upper thighs. Blood pressure was 120/80 mmHg.
Investigations: A fasting blood test showed normal lipid profile, liver function, glucose and insulin levels. Urinalysis gave normal results.
Management: The GP arranged to see the girl and her mother together and separately, initially every 3 weeks and then less often. Two visits to a local dietitian were arranged; a long waiting list made further follow-up impossible. The girl was encouraged to set her own goals for food and activity changes, which were discussed with the GP at each consultation. The family was supported in changing their eating patterns and television use. The dietitian looked with the girl at ways to recognise and deal with eating cues.
Course: Over time, the mother herself started to lose weight because of altered cooking practices and being more active. Water rather than soft drink was offered at the evening meal, biscuits and less-healthy snacks were no longer stored in the cupboards, and the whole family moved to eating more vegetables and having smaller meat portions at the evening meal. The girl ate something for breakfast each morning and started walking to and from school each day. She began tennis lessons and found an interest in tap dancing.
Ten months later, the girl’s weight was 69.3 kg, height 163.0 cm, BMI 26.1 kg/m2, and waist circumference 80 cm. She reported being fitter and said that she was greatly enjoying school and was no longer being bullied.
7: Case report — relapsing obesity in an adult
Presentation: A 42-year-old woman was referred for management of severe obesity and type 2 diabetes.
History: She had been thin as a child but started to gain weight after the birth of her first child, 20 years before. She had attempted weight loss several times previously, at one time losing 30 kg using a commercial weight-loss program, but always regained the weight.
Two years prior she was diagnosed with diabetes after presenting with polyuria and thrush. This was treated with metformin (1000 mg twice daily) and glibenclamide (5 mg once daily). She had a past history of hypercholesterolaemia and hypertension, treated with atorvastatin (40 mg in the morning) and ramipril (2.5 mg in the morning), and pre-eclampsia during one pregnancy. She had a maternal history of obesity and diabetes. She was a non-smoker. She was married with two daughters and was a full-time mature-age university student.
Examination: She appeared well. Weight was 114.5 kg, height 1.65 m (body mass index, 42.1 kg/m2, corresponding to obesity class III), waist circumference 125 cm, and hip circumference 131 cm. Pulse was regular at 98 beats/min, blood pressure was 130/90 mmHg. She had mild acanthosis nigricans around the neck and a few skin tags. Cardiorespiratory examination revealed no abnormalities. Pulses were difficult to feel. There was no evidence of microangiopathy.
Investigations: Serum levels were: glycosylated haemoglobin, 10.2% (reference range [RR], < 6.1%); total cholesterol, 4.8 mmol/L (RR, < 5.6 mmol/L); triglyceride, 3.3 mmol/L (RR, < 2.6 mmol/L); high-density lipoprotein (HDL) cholesterol, 0.86 mmol/L (RR, > 1.0 mmol/L); low-density lipoprotein (LDL) cholesterol, 3.34 mmol/L (RR, < 3.51 mmol/L); LDL/HDL cholesterol ratio, 3.9 (RR, < 3.6). Serum levels of electrolytes, urea and creatinine, and liver function results, were in the reference range.
Management: The patient said that she was ready to attempt weight loss as she understood that obesity was contributing to her diabetes, hypertension and dyslipidaemia. A diet and exercise regimen was discussed, and she was referred to a dietitian for help in calculating a 2 500 kJ (600 calorie) deficit diet. She undertook to try to increase her physical activity.
1-month review: The patient said she was struggling, as combining full-time study, part-time work and maintaining a household left little time for exercise and, although she tried to change her diet, she found it difficult not to snack. She said she had found it easier to diet when she was younger. Her diabetes had not improved.
Management: Given the patient’s medical comorbidities, it was worth considering more aggressive management of obesity.
Gastric banding was discussed. She had considered this after seeing it on television. However, she had no private insurance, and waiting lists at the few public hospitals that perform the operation are up to 5 years.
A medical strategy was adopted. She began a 3-month course of:
A very low energy diet (VLED), comprising a sachet of essential nutrients mixed with water three times daily (providing vitamins, minerals, amino acids, essential fatty acids and 1914 kJ [456 calories] per day). This was purchased over-the-counter from a pharmacist.
A bowl of vegetables or salad (to supply roughage) sprinkled with a teaspoon of olive oil (to contract the gall bladder) in the evenings.
She stopped taking glibenclamide, both to remove the risk of hypoglycaemia and to allow insulin levels to fall, producing mild ketosis to help control hunger. She was asked to have serum electrolytes, urea, creatinine and uric acid levels measured 6 weeks into the diet. She saw the dietitian fortnightly.
3-month review: After 3 months of the diet she had lost 17 kg, and blood glucose levels had improved dramatically, with glycosylated haemoglobin dropping to 7.9%.
Management: She was advised to phase out the VLED over the next 2 months and to adopt a low-fat, low-carbohydrate diet. However, as previous lost weight had always been regained, sibutramine (10 mg, mornings) was prescribed to assist weight maintenance. She reported it had a noticeable effect on hunger and satiety, but she was struggling financially because of its cost ($120 per month).
8: Drugs currently available to treat obesity
Drug |
Function |
Side effects |
Weight loss v placebo* |
Comments |
|||||||
Phentermine, diethylpropion |
Noradrenergic agonists that stimulate adrenergic pathways, suppressing hunger |
Insomnia, dry mouth, nervousness, irritability, increase in blood pressure, angina |
6.3 kg v 2.8 kg |
No studies supporting long-term use. |
|||||||
Orlistat |
Inhibits intestinal lipase, reducing fat absorption by 30% |
Steatorrhoea, bloating, faecal leakage, especially if excess fat is eaten |
7.1 kg v 5.0 kg |
Unpleasant side effects may encourage adherence to low-fat diet. |
|||||||
Sibutramine |
Metabolites inhibit serotonin- and noradrenaline-reuptake, enhancing satiety and suppressing hunger |
Dry mouth, constipation, insomnia, increase in blood pressure (usually small) and heart rate |
5.3 kg v 1.8 kg |
Should be avoided in patients with uncontrolled hypertension or angina. Effects on cardiovascular endpoints (stroke, myocardial infarction) unknown. |
|||||||
Fluoxetine |
Selective serotonin-reuptake inhibitor that inhibits hunger |
Seizures, nausea, insomnia, sexual dysfunction |
4.1 kg v 0.8 kg |
Not approved for treating obesity but drug of choice for treating depression in overweight patients. |
|||||||
* From Haddock et al, meta-analysis of weight loss studies.36 |
|||||||||||
9: Laparoscopic gastric banding
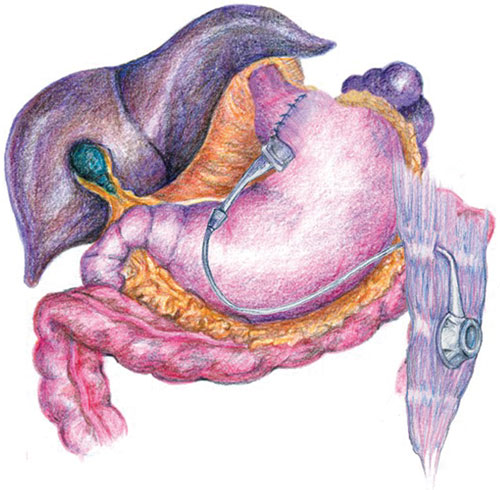
An adjustable band is sutured around the very upper stomach. The restriction on the stomach can be modified by adding or removing saline from a reservoir on the rectus sheath. This procedure can be performed periodically in the office. (Illustration courtesy of Dr J Dixon, Monash University, Centre for Obesity Research and Education, Melbourne, VIC, and InaMed Health, Santa Barbara, USA)
- Joseph Proietto1
- Louise A Baur2
- 1 Department of Medicine, Repatriation Hospital, Melbourne, VIC.
- 2 Children's Hospital at Westmead, Sydney, NSW.
J P is chair of the medical advisory board for Optifast (Novartis) and a member of the medical advisory boards for Orlistat (Roche) and Sibutramine (Abbott).
- 1. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998; 22: 1164-1171.
- 2. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Geneva, 3-5 June 1997. Geneva: WHO, 1998.
- 3. Ko GT, Tang J, Chan JC, et al. Lower BMI cut-off value to define obesity in Hong Kong Chinese: an analysis based on body fat assessment by bioelectrical impedance. Br J Nutr 2000; 85: 239-242.
- 4. Swinburn BA, Ley SJ, Carmichael HE, et al. Body size and composition in Polynesians. Int J Obes Relat Metab Disord 1999; 23: 1178-1183.
- 5. Han TS, Van Leer EM, Seidell JC, et al. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 1995; 311: 1401-1405.
- 6. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000; 8: 1-27.
- 7. National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults. Canberra: NHMRC, 2003.
- 8. Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240-1243.
- 9. Maffeis C, Pietrobelli A, Grezzani A, et al. Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res 2001; 9: 179-187.
- 10. Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr 1999; 70: S149-S156.
- 11. National Heart Foundation of Australia. Risk factor prevalence study No 1. 1980 Canberra: NHF, 1980.
- 12. Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427-432. <MJA full text>
- 13. Magarey AM, Daniels LA, Boulton TJ. Prevalence of overweight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard international definitions. Med J Aust 2001; 174: 561-564. <MJA full text>
- 14. Stunkard AJ, Harris JR, Pedersen NL, et al. The body-mass index of twins who have been reared apart. N Engl J Med 1990; 322: 1483-1487.
- 15. Sorensen TI, Holst C, Stunkard AJ, et al. Correlations of body mass index of adult adoptees and their biological and adoptive relatives. Int J Obes Relat Metab Disord 1992; 16: 227-236.
- 16. Clement K, Boutin P, Froguel P. Genetics of obesity. Am J Pharmacogenomics 2002; 2: 177-187.
- 17. Robinson TN. Television viewing and childhood obesity. Pediatr Clin North Am 2001; 48: 1017-1025.
- 18. Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001; 357: 505-508.
- 19. Jeffery RW. Minnesota studies on community-based approaches to weight loss and control. Ann Intern Med 1993; 119: 719-721.
- 20. Campbell K, Waters E, O’Meara S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev 2002, CD001871.
- 21. Economos CD, Brownson RC, DeAngelis MA, et al. What lessons have been learned from other attempts to guide social change? Nutrition Reviews 2001; 59: S40-S56.
- 22. Ludwig DS, Pereira MA, Kroenke CH, et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA 1999; 282: 1539-1546.
- 23. Brand-Miller J, Foster-Powell K, Colagiuri S. The new glucose revolution. 2nd ed. Sydney: Hodder Headline Australia, 2003.
- 24. Harvey EL, Glenny A-M, Kirk SFL, Summerbell CD. Improving health professionals' management and the organisation of care for overweight and obese people (Cochrane Review). In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley and Sons, Ltd.
- 25. Epstein LH, Valoski A, Wing RR, et al. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA 1990; 264: 2519-2523.
- 26. Epstein LH, Cluss PA. A behavioral medicine perspective on adherence to long-term medical regimens. J Consult Clin Psychol 1982; 50: 950-971.
- 27. Golan M, Weizman A, Apter A, et al. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr 1998; 67: 1130-1135.
- 28. Summerbell CD, Waters E, Edmunds L, et al. Interventions for treating obesity in children (protocol for a Cochrane review). The Cochrane Library, 2003. Oxford: Update Software.
- 29. Brownell KD, Kelman JH, Stunkard AJ. Treatment of obese children with and without their mothers: changes in weight and blood pressure. Pediatrics 1983; 71: 515-523.
- 30. Saelens BE, Sallis JF, Wilfley DE, et al. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res 2002; 10: 22-32.
- 31. National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in children. Canberra: NHMRC, 2003. Available at: www.obesityguidelines.gov.au (accessed Mar 2004).
- 32. Burry JN. Obesity and virtue. Is staying lean a matter of ethics? Med J Aust 1999; 171: 609-610. <MJA full text>
- 33. Scheen AJ. Results of obesity treatment. Ann Endocrinol (Paris) 2002; 63: 163-170.
- 34. Geldszus R, Mayr B, Horn R, et al. Serum leptin and weight reduction in female obesity. Eur J Endocrinol 1996; 135: 659-662.
- 35. Montague CT, Farooqi IS, Whitewhead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997; 387: 903-908.
- 36. Haddock CK, Poston WS, Dill PL, et al. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes 2002; 26: 262-273.
- 37. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27: 155-161.
- 38. James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet 2000; 356: 2119-2125.
- 39. Dixon JB, O’Brien PE. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. Am J Surg 2002; 184: 51S-54S.
- 40. Sjostrom L. Obesity, diabetes and other cardiovascular risk factors: lessons from SOS. Int J Obes 2000; 24: S7.
- 41. Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care 2002; 25: 358-363.
- 42. Allen JW, Coleman MG, Fielding GA. Lessons learned from laparoscopic gastric banding for morbid obesity. Am J Surg 2001; 182: 10-14.





Abstract
Improved understanding of the regulation of body weight and activity is leading to new treatment strategies