Pressure ulcers are preventable adverse events that are both common and costly. A national survey in the United States reported a prevalence of 14.8% across 365 acute hospitals.1 A pressure ulcer of Stage 2 or higher has been calculated to increase patients’ costs by a factor of 2.7,2 while the annual cost of pressure ulcers to the American healthcare system is estimated at $US3.6 billion.3
Many types of pressure-relieving support surfaces are available,4,5 but a Cochrane systematic review found that the only surfaces that consistently outperformed the standard hospital mattress in reducing the incidence of pressure ulcers were high-specification foam mattresses.4 However, the review also stated that most interventional studies of pressure-relieving support surfaces were seriously underpowered or had other methodological flaws.4
In the 1960s and 1970s, sheepskins were reported to help prevent pressure ulcers,6-10 but recent reviews have found inconclusive evidence to support their use.4,5,11,12 In 1998, the CSIRO (Commonwealth Scientific and Industrial Research Organisation) introduced a new high-performance medical sheepskin, the Australian Medical Sheepskin. This has a denser and higher wool pile and can withstand multiple washes at 80°C, representing a significant advance in leather technology.13 A recent randomised controlled trial found that use of this sheepskin reduced the incidence of pressure ulcers by 68% (95% CI, 54%–84%) compared with standard practice in 297 elderly orthopaedic patients.14
To extrapolate these findings to a broader hospital population, we conducted an open-label randomised controlled clinical trial of the effectiveness, relative to usual nursing care, of the Australian Medical Sheepskin in reducing the incidence of pressure ulcers in general hospital inpatients.
The trial was conducted at the Royal Melbourne Hospital, a general teaching hospital with about 360 inpatient beds in Melbourne, Victoria. The trial was approved by the Clinical Research and Ethics Committee of the Royal Melbourne Hospital Research Foundation.
All patients who were admitted to the hospital between 12 June and 30 November 2000 were eligible for the trial if they were at low to moderate risk of developing a pressure ulcer on the Braden Pressure Ulcer Risk Assessment Scale.15 This validated scale is based on mobility, activity, sensory perception, nutrition, exposure to moisture, shear and friction.
Patients were excluded from the trial if they:
were assessed as at “no risk” (requiring no intervention) or “high risk” (requiring more complex interventions);
had any pre-existing pressure ulcer;
were less than 18 years of age;
had an expected length of stay less than 48 hours; or
had darkly pigmented skin, making a Stage 1 pressure ulcer difficult to detect.
Participants were recruited by one of five clinical nurse specialists employed as research nurses. These nurses attended the emergency department two to three times daily during morning and afternoon shifts on weekdays and morning shifts on weekends, and recruited as many patients as possible during these hours. The nurses also assessed patients attending pre-admission clinics for targeting on admission.
All patients were assessed for risk of pressure ulcers within 24 hours of admission using the Braden scale.15 Formal informed consent was obtained from patients for participation in the trial.
Immediately after risk assessment, patients were randomly allocated to receive either the sheepskin or standard treatment, using numbered cards in individually sealed opaque envelopes; blocks of 16 envelopes (eight of each group) were shuffled before use.
The Australian Medical Sheepskin is a leather-backed sheepskin with a dense, uniform, 25 mm natural wool pile. The sheepskin is used without covering as a partial mattress overlay and is specifically designed to reduce pressure, minimise shear and friction and absorb moisture.13 It meets Australian Standard AS4480.1-1998.16
A sheepskin overlay was fitted to the patient’s bed immediately after allocation to the sheepskin group, and a sheepskin remained in place until the patient was discharged. Sheepskins were changed on Mondays, Wednesdays and Fridays or when soiled, and were laundered to the specifications of Australian Standard AS4480.1-1998 to achieve thermal disinfection.16 Pressure points not covered by the sheepskin were protected with a second sheepskin or specific sheepskin elbow and heel protectors. Patients in the sheepskin group received usual nursing care, including repositioning, as determined by ward staff.
The referent group used any other pressure-relieving device or prevention strategy deemed appropriate by ward nursing staff, comprising standard hospital mattress and sheet, with or without other low-technology constant-pressure-relieving devices and repositioning as determined by nursing staff.
As it was logistically impossible to blind patients, ward staff and research nurses to the treatment group, this was an open-label, unblinded trial.
Research nurses assessed each participant daily for pressure-ulcer risk as described previously, and for skin integrity. Any patient whose risk increased to “high” (Braden score < 12) for 48 hours was no longer followed up for pressure-ulcer endpoints for this trial.
Patients were assessed for new ulcers using a standardised protocol and the operational definitions of the US Agency for Health Care Policy and Research (Box 1).12 A Stage 1 ulcer was diagnosed if non-blanching erythema (using finger-point pressure) was still present after 30 minutes of pressure relief to the affected area.14 Suspected ulcers were assessed by a second research nurse. Research nurses notified ward staff if a pressure ulcer was observed.
Interobserver reliability was measured using blinded observation on 45 patients in pairs with four clinical nurses. For total Braden score, the intraclass correlation estimate was r, 0.89 (95% CI, 0.82–0.96). During the trial, agreement between paired observers recording erythema or Stage 1 ulcers was 94% (32/34 pairs) with weighted κ = 0.90.
We calculated pressure-ulcer incidence rates in each group as the ratio of the number of new pressure ulcers to the number of bed-days observed. Confidence intervals were inferred around each point estimate using Poisson probability distribution.17 The efficacy of the sheepskin relative to usual care was assessed by the ratio of incidence rates.
Kaplan–Meier survival functions were used to describe time in days to development of first ulcer, and relative ulcer-free survival was estimated using Cox regression methods.17 Patients were censored on discharge or progression to high risk status.
A previous Australian survey in a general hospital population reported an ulcer incidence rate of 50 per 2500 patient-days, or 2% per day.18 We postulated that a twofold reduction in pressure ulcer incidence was feasible and cost-effective. For a test at a significance level of 5% to distinguish between expected incidence rates of 2% per day and 1% per day with 80% power, at least 70 new pressure ulcers (across both groups) are required.19 No interim analysis was planned or performed. After 3289 bed-days of observation, we had recorded 85 new pressure ulcers and ceased recruiting.
The flow of participants through each stage of the trial is shown in Box 2; 539 of 1900 potential participants were randomly allocated. Of these, 441 received the allocated intervention. All 441 were followed up to the endpoints of discharge, death, withdrawal based on clinical decision by ward staff, change in pressure-ulcer risk status to high risk, or patient request.
Baseline demographic and clinical characteristics of the 441 patients are shown in Box 3. The sheepskin and referent groups differed substantially only by admission type, as there were more emergency admissions in the sheepskin group.
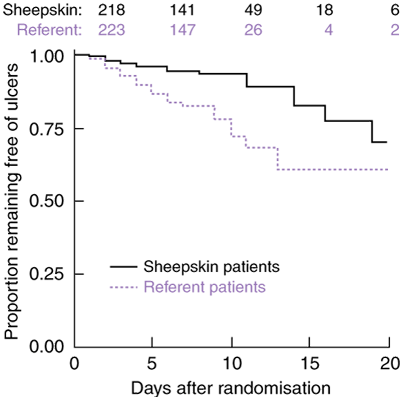
Primary outcome measures are shown in Box 4. The crude incidence rate of pressure ulcers in the sheepskin group was 0.42 times that in the referent group (95% CI, 0.26–0.67), implying a reduction in pressure-ulcer risk of more than 50%. A consistent, but not statistically significant, result was obtained by restricting analysis to Stage 2 ulcers, for which the crude incidence rate ratio was 0.54 (95% CI, 0.24–1.16). No Stage 3 or 4 ulcers were seen in either group.
Results showed that the sheepskin may prevent, on average, one new Stage 1 or 2 ulcer every 46 bed-days of use (attributable risk, 3.7 – 1.6 = 2.15 per 100 bed-days; therefore, number of bed-days needed to treat to prevent one ulcer = 100/2.15 = 46.4). The difference in cumulative incidence risk between the sheepskin and referent groups was 6.9%, giving an estimated average number of patients needed to treat of 14.4 to prevent the development of pressure ulcers in one patient.
Kaplan–Meier survival curves for time to onset of first ulcer (Box 5) show separation between the sheepskin and referent groups (P < 0.001, log-rank test). A hazard ratio of 0.39 (95% CI, 0.22–0.69), estimated using Cox proportional hazards regression, confirmed the magnitude and direction of the rate ratio of crude incidence rates. After 20 days, few patients remained in either group, invalidating further comparison.
Seven patients died during follow-up (2 sheepskin, 5 referent), and a further eight progressed to “high risk” for pressure ulcers for more than 48 hours while under observation (7 sheepskin, 1 referent). After review, none of these events was considered attributable to the intervention. Ten patients in the sheepskin group complained about its comfort (“too hot”, 6; sensitive to the wool surface, 2; “uncomfortable”, 2) and requested its removal.
We found that use of the Australian Medical Sheepskin as a mattress overlay reduced the incidence of Stage 1 or 2 pressure ulcers by 58% (1.6, compared with 3.7 pressure ulcers per 100 bed-days) compared to standard nursing care in general hospital inpatients at low to moderate risk of developing a pressure ulcer. However, comparing incidence rates alone is not sufficient to establish effectiveness of the sheepskin, as some patients developed multiple ulcers. For individual patients, the risk of developing a pressure ulcer in the sheepskin group was 40% less than the risk in the referent group (9.6%, compared with 16.6%).
As this study was an open-label, unblinded trial, it had potential biases. Most prominent is observer bias in diagnosing pressure ulcers, particularly Stage 1 ulcers, which are notoriously difficult to diagnose. However, the research nurses followed a strict protocol in diagnosing these ulcers, and their actions were recorded on the data collection charts and checked. Furthermore, an analysis of Stage 2 ulcers alone also found a reduction of 46% in their incidence in the sheepskin group relative to the referent group.
There was also potential for observer bias in assessing patient risk. Indeed, the number of patients who were assessed as high risk immediately after randomisation and failed to receive the allocated intervention was greater in the sheepskin group than in the referent group (15 versus 8). However, risk was assessed with an objective reliable instrument,15 so that bias was unlikely. Although more patients in the sheepskin group were excluded during the trial after they became “high risk” (7 versus 1 in the referent group), all ulcers observed up to the time of exclusion were included in the analysis (3 in the sheepskin and 1 in the referent group). On balance, the total number of exclusions was small and unlikely to have influenced incidence rate ratios.
As well as observer bias, nursing care may have differed between the two groups, with patients in the sheepskin group receiving either additional care (because of a perception that they were “at risk”) or, alternatively, less pressure-relief care (because they already had a therapeutic device in situ). Either possibility is difficult to confirm or disprove. The length of the study (25 weeks) and number of clinical units involved (14) might be expected to minimise any differences in nursing care between the two groups.
The results of this study extend the previous findings in an elderly orthopaedic population14 to the general adult hospital population. The Royal Melbourne Hospital is a general adult teaching hospital that serves a broad population. We believe our sample is representative of the general adult population found in most tertiary hospitals,20 and that our results can be generalised to most similar hospitals across Australia.
The results suggest that an Australian Medical Sheepskin pressure-relieving support surface may prevent one new Stage 1 or 2 ulcer every 46 bed-days of use in general hospital patients at low to moderate risk. The estimated number of patients needed to treat to prevent ulcers in one patient was 14.4, similar to the number found in orthopaedic patients.14
Our results apply when the sheepskin is used for prophylaxis from the time of admission. We did not investigate the efficacy of the sheepskin in the presence of pre-existing pressure ulcers. Although the sheepskin may initially add to the cost of patient care, it has the potential to reduce the incidence of pressure ulcers in general hospital patients.
1: Classification of pressure ulcers11
Stage 1: Persistent non-blanching erythema; epidermis remains intact.
Stage 2: Partial thickness loss of skin layers involving the epidermis and possibly penetrating into, but not through, dermis. The ulcer is superficial and presents clinically as an abrasion, blister or shallow crater. The wound base is moist, pink and free of necrotic tissue.
Stage 3: Full thickness skin loss involving damage or necrosis of subcutaneous tissue that may extend down to, but not through, the underlying fascia. The ulcer presents clinically as a deep crater with or without undermining of adjacent tissue.
Stage 4: Full thickness skin loss with extensive destruction, tissue necrosis or damage to muscle, bone, or supporting structures. Undermining and sinus tract may also be present.
2: Flow of participants
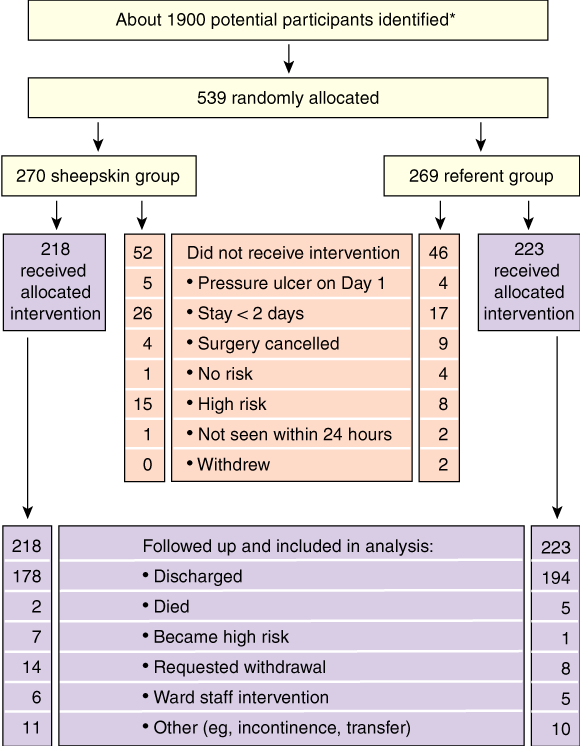
* About 1300 potential participants either were not approached or did not meet inclusion criteria.
3: Baseline characteristics of 441 patients at entry
|
Sheepskin (n = 218) |
Referent (n = 223) |
|||||||||
Mean age (range) |
63.2 (18–97) |
61.1 (18–99) |
|||||||||
Sex (% female) |
49% |
52% |
|||||||||
Emergency admission |
51% |
43% |
|||||||||
Medical specialty |
|
|
|||||||||
General surgery |
12% |
13% |
|||||||||
Orthopaedics |
19% |
17% |
|||||||||
Neurosurgery |
21% |
24% |
|||||||||
General medical |
22% |
17% |
|||||||||
Other surgical* |
13% |
19% |
|||||||||
Other medical† |
13% |
10% |
|||||||||
Mean score for pressure-ulcer risk (range)‡ |
15.7 (13–18) |
15.9 (13–18) |
|||||||||
* Including plastic, cardiothoracic, vascular, renal and urological surgery. † Including oncology, endocrinology, rheumatology, cardiology and gastroenterology. ‡ Braden score for pressure-ulcer risk: high risk (< 12); moderate risk (13–14); low risk (15–18).15 |
|||||||||||
4: Outcomes in 441 patients randomly allocated to sheepskin or standard (referent) treatment
Outcome |
Sheepskin (n = 218) |
Referent (n = 223) |
|||||||||
Total bed-days observed |
1728 |
1561 |
|||||||||
Mean bed-days observed/participant |
7.9 |
7.0 |
|||||||||
Number of participants with ulcer(s) |
21 |
37 |
|||||||||
Total number of ulcers |
27 |
58 |
|||||||||
Cumulative incidence risk (%) (95% CI) |
9.6% |
16.6% |
|||||||||
Risk ratio (95% CI) |
0.58 (0.35–0.96) |
1.0 |
|||||||||
Incidence rate per 100 bed-days (95% CI) |
1.6 (1.0–2.3) |
3.7 (2.8–4.8) |
|||||||||
Incidence rate ratio (95% CI) |
0.42 (0.26–0.67) |
1.0 |
|||||||||
Number of Stage 2 ulcers (% of all ulcers) |
12 (44%) |
20 (34%) |
|||||||||
Odds ratio of Stage 2 v Stage 1 ulcer (95% CI) |
1.52 (0.54–4.3) |
1.0 |
|||||||||
Received 15 April 2003, accepted 23 October 2003
- Damien J Jolley1
- Robyn Wright2
- Sunita McGowan3
- Mark B Hickey4
- Kenneth C Montgomery5
- Don A Campbell6
- Rodney D Sinclair7
- 1 School of Health Sciences, Deakin University, Melbourne, VIC.
- 2 School of Nursing, University of Melbourne, Melbourne, VIC.
- 3 Fremantle Hospital and Health Service, Fremantle, WA.
- 4 CSIRO Leather Research Centre, Melbourne, VIC.
- 5 Clinical Epidemiology Unit, Royal Melbourne Hospital, Melbourne, VIC.
- 6 University of Melbourne (St Vincent’s Hospital), Melbourne, VIC.
We wish to thank Donna McVean, Carole Meijs, Adriana Tiziani, Rachael Duncan, Margot Yeomans (Royal Melbourne Hospital), Jacinta Wassenberg (CSIRO), Carl Gibbons (Deakin University), and Clare Murphy and Trang Vu (Victorian Public Health Trainee Scheme).
Funding for this study was provided by a project grant from the National Health and Medical Research Council of Australia, and by the CSIRO Textile and Fibre Technology, Leather Research Centre.
KCM and MBH are employees of CSIRO Textile and Fibre Technology, Leather Research Centre, which coordinated the development of the Australian Standard 4480.1-1998 for the Australian Medical Sheepskin.
- 1. Amlung S, Miller W, Bosley L. The 1999 national pressure ulcer prevalence survey: a benchmarking approach. Adv Skin Wound Care 2001; 14: 297-302.
- 2. Allman R, Goode P, Burst N, et al. Pressure ulcers, hospital complications and disease severity: impact on hospital costs and length of stay. Adv Wound Care 1999; 12: 22-30.
- 3. Beckrich K, Aronovitch S. Hospital-acquired pressure ulcers: a comparison of costs in medical vs. surgical patients. Nurs Econ 1999; 17: 263-271.
- 4. Cullum N, Deeks J, Sheldon T, et al. Beds, mattresses and cushions for pressure sore prevention and treatment (Cochrane Methodology Review). The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley.
- 5. Australian Wound Management Association. Clinical practice guidelines for prediction and prevention of pressure ulcers. Perth: Cambridge Media, 2001.
- 6. Denne W. An objective assessment of the sheepskins used for decubitus sore prophylaxis. Rheumatol Rehabil 1979; 18: 23-29.
- 7. Ewing M, Garrow C, McHugh N. A sheepskin as a nursing aid. Lancet 1961; 30: 1447-1448.
- 8. Ewing M, Garrow C, Presely T, et al. Further experiences in the use of sheepskins as an aid in nursing. Med J Aust 1964; 2: 139-141.
- 9. Gorick G. Sheepskin boots in the prevention of pressure sores. Nurs Times 1965; 61: 1749-1751.
- 10. Laubscher N. A statistical evaluation of the effectiveness of medical sheepskins for the prevention of pressure sores. S Afr Med J 1966; 40: 599-601.
- 11. Bergstrom N, Allman R, Carlson C, et al. Pressure ulcers in adults: prediction and prevention. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, 1992.
- 12. Royal College of Nursing. Pressure ulcer risk assessment and prevention. London: RCN, 2000.
- 13. Commonwealth Scientific and Industrial Research Organisation. Australian medical sheepskins research paper. Available at: www.tft.csiro.au/leather/amedskins.html (accessed Nov 2003).
- 14. McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Primary Intention 2000; 8: 127-134.
- 15. Bergstrom N, Braden B, Kemp M, et al. Predicting pressure ulcer risk: a multisite study of pressure validity of the Braden Scale. Nurs Res 1998; 47: 261-269.
- 16. Australian Standards International. AS4480.1-1998. Textiles for healthcare facilities and institutions — medical sheepskins — product specifications and testing Sydney: Australian Standards Australia, 1998.
- 17. Clayton D, Hills M. Statistical models in epidemiology. Oxford: Oxford University Press, 1993.
- 18. McGowan S, Hensley L, Maddock J. Monitoring the occurrence of pressure ulcers in a teaching hospital: a quality improvement project. Primary Intention 1996; 4: 9-16.
- 19. Smith P, Morrow R, editors. Methods for field trials of interventions against tropical diseases: A toolbox. Oxford: Oxford University Press, 1991.
- 20. Australian Institute of Health and Welfare. Australian hospital statistics 1999-00. Canberra: AIHW, 2001. (Health Services Series No 17, AIHW Catalogue No. HSE 14.)





Abstract
Objective: To estimate the effectiveness of a new high-performance Australian medical sheepskin (meeting Australian Standard 4480.1-1998) in preventing pressure ulcers in a general hospital population at low to moderate risk of these ulcers.
Design: Open-label randomised controlled clinical trial.
Setting: A large metropolitan teaching hospital in Melbourne, Victoria, in 2000.
Participants: 441 patients aged over 18 years admitted between 12 June and 30 November 2000, with expected length of stay over 2 days and assessed as at low to moderate risk of developing pressure ulcers.
Intervention: Patients were randomly allocated to receive a sheepskin mattress overlay for the duration of their hospital stay (218 patients) or usual treatment, as determined by ward staff (referent group, 223 patients).
Main outcome measures: Incidence rate and cumulative incidence of pressure ulcers, assessed daily throughout hospital stay.
Results: 58 patients developed pressure ulcers (sheepskin group, 21; referent group, 37). Cumulative incidence risk was 9.6% in the sheepskin group (95% CI, 6.1%–14.3%) versus 16.6% in the referent group (95% CI, 12.0%–22.1%). Patients in the sheepskin group developed new pressure ulcers at a rate less than half that of referent patients (rate ratio, 0.42; 95% CI, 0.26–0.67).
Conclusions: The Australian Medical Sheepskin is effective in reducing the incidence of pressure ulcers in general hospital inpatients at low to moderate risk of these ulcers.