Osteoarthritis (OA) affected over 1.2 million Australians in 19951 and is associated with both substantial loss of quality of life and high management costs. In 2001, these costs were estimated at $1090 million.2
There is a wide range of options for managing OA. These include prescription drugs, over-the-counter medications, exercise and strength training, surgery, patient education and complementary therapies. Recognising that distortions in funding arrangements for the delivery of healthcare result in highly disparate cost-effectiveness ratios,3-5 logic suggests there are opportunities for health gain in redistributing resources from services that perform poorly (high cost per unit of benefit) to those that perform better (low cost per unit of benefit). Priority-setting models are designed to identify such desirable resource shifts, and one model that has been found to be appropriate for national or regional decision-making is the Health-sector-Wide (HsW) disease-based model.6,7 This model, like the Quality Adjusted Life Year (QALY) league table approach and Program Budget Marginal Analysis, involves comparing marginal cost-effectiveness ratios of possible interventions.7 The model also insists on objective evidence for determining effectiveness, a defined process for selecting interventions to ensure comprehensiveness, and a means for staging the analysis through diseases and disease stages. The model was recently applied to investigating how the disease burden of OA might be reduced by adjusting the health service mix.2
Applying the model involved three broad research tasks:
identifying all potential interventions (service types) for preventing and managing OA and, through a literature review, selecting a subset for inclusion in the model;
conducting economic evaluations of the selected interventions, using published evidence on outcomes and costs; and
comparing performance of the interventions (primarily in terms of cost-effectiveness) and developing policy recommendations on desirable resource shifts.
An advisory panel of clinicians, health department officers, consumers and consumer organisation representatives (see Acknowledgements) was established to support the research team, ensuring appropriate coverage of the literature and application of the model.
The selection of management options to be included in the HsW model aims for comprehensive coverage of treatment modalities, delivery settings, disease stage and population subgroups, including common approaches to management and more innovative approaches. Reliance on objective evidence requires that published trial data contain, at a minimum, a precise program description and quantitative evidence of effectiveness derived from an acceptable research design, and, preferably, health endpoints, a usual-care or placebo control, and a suitable follow-up period. In the OA study, six standard search engines, including MEDLINE, PubMed and CINAHL, were used to identify potential interventions. Cochrane Reviews were used when available, and 19 interventions were selected with input from the advisory panel (Box 1).
The variety of disease-specific and generic instruments that have been used to report the effectiveness of OA treatments makes it difficult to compare published studies. HsW modelling required a means to compare each intervention on the same scale. Ideally, the method for doing this should also enable global comparisons, and combine quality of life and mortality. Health-Related Quality of Life, based on “utility” and scaled between 0 (death) and 1 (best possible health), meets this requirement. Outcomes expressed as utility values can be combined with time lived in particular health states and added to mortality rate differentials to calculate effectiveness in quality-adjusted life-years (QALYs). In this way, the performance of all interventions can be measured in the same metric (cost/QALY) and directly compared. Utility values can be obtained from a multi-attribute utility instrument such as the European EQ-5D30 or the Australian Assessment of Quality of Life (AQoL) instrument.31,32 Conversion of the SF-3633 to a utility scale has been the subject of ongoing research, but the initial algorithm appeared to truncate at the severe end of the scale and was therefore unsuitable for our purposes.34,35 While this conversion has since been revised, it requires access to the full SF-36 dataset, which is an impediment to its use.36 The use of disability weights to calculate disability-adjusted life-years (DALYs) was also rejected, as the published disability weights for OA describe only three levels, which did not map onto trial data.37
For this study, commonly used OA outcome scales were converted into a utility-equivalent scale. The technique, termed “transfer to utility”, or TTU, involved administering the AQoL alongside common OA outcome instruments such as the SF-36, a visual analogue scale (VAS) for pain, and the Western Ontario and McMaster Arthritis Index38 in 303 people with OA. Participants were recruited from rheumatology clinics, orthopaedic waiting lists and the Arthritis Foundation of Victoria to ensure a wide range of severity of OA was captured. The survey was approved by the Royal Melbourne Hospital Human Ethics Committee.
Equivalent utility values (based on the AQoL) for the selected outcome instruments were estimated from these data by means of multiple regression analysis. These relationships were applied to outcomes reported in published studies for both the intervention and control cohorts to estimate utility gain attributable to interventions.
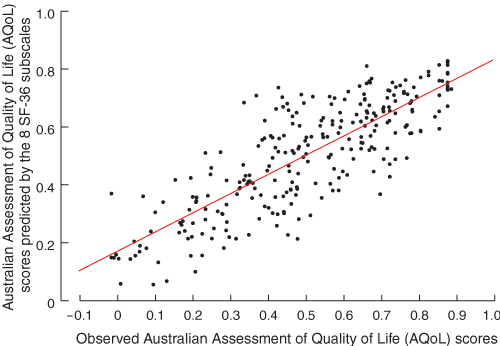
The technique can be illustrated by applying it to total hip replacement (THR), where the reported outcomes are the SF-36 subscale scores, measured before and after surgery. A multiple linear regression model of the AQoL (dependent variable) was constructed as a function of the eight subscales of the SF-36. The relationship between the observed and predicted scores is shown in Box 2, and shows the good explanatory power of the model (adjusted R2 = 0.66; P < 0.001). Separate weights were obtained for hip OA (R2 = 0.70; P < 0.001) and knee OA (R2 = 0.63; P < 0.001). Box 3 shows hip-specific parameters and the estimation of utility-equivalent outcomes from the chosen THR study,28 showing a utility gain 12 months after surgery of 0.304. Further details of the “transfer to utility” technique are described elsewhere.2
Future impacts (costs and benefits) were calculated where relevant and when evidence was available. For THR and total knee replacement (TKR) surgery, benefits and costs (including costs for revision surgery) were modelled over 15 years, knee bracing was modelled over 18 to 36 months and exercise over 12 months. Primary care was modelled over 20 years, with estimated mortality based on all-cause mortality, and quality-of-life gain based on the reduced incidence of OA.
Because there has been debate about the desirability of discounting health,39 we derived two sets of cost-effectiveness estimates, using discount rates on health benefits of 5% per annum and zero, but costs always discounted at 5% per annum. In the United Kingdom, future life-years are discounted at 1.5%–2.0% per annum, and future costs at 6% per annum (the status until 2003, with the revised standard discounting of costs and benefits at 3.5% per annum),40,41 while the Australian Pharmaceutical Benefits Advisory Committee guidelines for listing of drugs on the Pharmaceutical Benefits Scheme (PBS) suggest costs and benefits be discounted at 5% per annum.42
Costs included resources applied to the intervention and to the management of treatment side effects, and, for primary prevention, estimated savings in “downstream” healthcare service use. Intervention costs were calculated as the product of program inputs, based on descriptions of interventions, multiplied by current published unit costs (such as the PBS, the Medicare Schedule, Australian National Diagnosis Related Groups cost-weights, and standard charge-out rates for health-professional groups). Further detail is reported elsewhere.2
For each intervention, values for incremental cost/QALY were calculated as the quotient of differential program cost (adjusted for any cost savings) and estimated utility benefit (relative to a placebo or usual care comparator), and then cost/QALY estimates were compared.
While the study takes a societal perspective, the scope of the costs and benefits included was limited by the data available. Also, published trial data (and study resources) did not allow subgroup analysis, which is ideal for comparing marginal populations within marginal programs (ie, those with the capacity to gain most or lose least from the addition or removal of services). Key assumptions are specified in Box 4. Univariate sensitivity analyses were performed, using alternative values for trial outcomes, period of benefit and discounting of future health gains or losses. The level of certainty in outcome and costs data was also noted.
Results are reported as utility benefit, cost per individual and cost/QALY in Box 5.
Surgery: The most effective intervention modelled was THR. The estimated utility gain from THR was 0.305. This indicates a substantial improvement in quality of life, considerably greater than that associated with other interventions modelled. As benefits persist for several years (except for the 12%–20% of patients expected to receive revision surgery),43,44 estimated QALY gain over a lifetime from THR was 3.52 per hip (adjusting for expected surgical mortality of 1/1000 and all-cause mortality in the cohort), and 2.086 per knee for TKR. At a weighted cost of primary and revision surgery of $16 000–$17 000 per case, cost/QALY for THR was $5000 (future benefits undiscounted) or $7000 (discounted at 5%), and, for TKR, a cost/QALY of $8000 (future benefits undiscounted) or $11 000 (discounted at 5%). Cost/QALY was not calculated for arthroscopy in knee OA, as recent randomised controlled trials (RCTs) suggest that the treatment is equivocal.29,45
Non-steroidal anti-inflammatory drugs (NSAIDs): Non-specific NSAIDs and COX-2 NSAIDs were found to perform similarly in terms of outcomes and side effects, based on seminal trials and a report by the United States Federal Drugs Administration which interpreted side-effect data.46 NSAIDs modelled were diclofenac, naproxen and celecoxib, using doses reported in the key trials. Based on three placebo/paracetamol-controlled trials,22-24 estimated mean utility benefit compared with placebo was 0.043. This benefit was offset by excess mortality equal to 0.044 or 0.029 QALYs (future life-years undiscounted or discounted at 5% per annum, respectively). Net QALY gain was close to zero or negative if future life-years from current deaths were undiscounted and 0.014 if discounted at 5% per annum, resulting in a wide estimate of cost/QALY from $15 000 to “infinity” for non-specific NSAIDs and between $33 000 and infinity for COX-2-specific NSAIDs. In most scenarios, COX-2 NSAIDs are dominated by non-specific NSAIDs.
Primary prevention: Because of the absence of RCTs for the primary prevention of OA through weight loss (or other means), this model used epidemiological data on the prevalence of OA and obesity, incorporating evidence of the effectiveness of weight loss interventions.1,8-10,12 Cost/QALY estimates ranged from $2000 to $48 000, depending on the type of program. But, in the absence of intervention trials, this analysis is speculative and requires testing through intervention trials. Primary prevention through modifying other risk factors, such as recreational or work-related injury, could not be established owing to lack of evidence.
Patient education: Although community-based education programs are widely applied, there is little published quantitative evidence. Group programs, including those run by lay leaders13,14 and health professionals,15,16 were reviewed, but cost/QALY estimates could not be generated as inconsistent findings made the evidence for effectiveness difficult to interpret.
Knee brace: Specifically manufactured and fitted knee braces for people with knee OA were found to be effective and cost-effective. Cost/QALY was $4000–$12 000, depending on the length of time the brace was worn.17
Exercise and strength training: Intensive exercise and strength training based in primary care20 or outpatient clinics21 was highly cost-effective ($3000–$15 000/QALY) and somewhat less cost-effective for an intensive home-based program ($10 000–$34 000/QALY).19 The evidence for the effectiveness of a less intensive home-based program was equivocal.18
Other pharmacotherapies: Topical capsaicin (for knee, hand, elbow and ankle OA) and glucosamine sulfate were found to be cost-effective at less than $5000/QALY. These drugs had equivalent efficacy to NSAIDs, with no evidence of excess morbidity or mortality.25,26,47 The performance of avocado/soy unsaponifiables was unclear because of conflicting results from the small number of published reports.48 Results for these therapies should be considered provisional, as they are based on only a few RCTs. However, recent trials lend support to a role for glucosamine sulfate.49,50
An important contribution of this priority-setting exercise is the development of an empirically driven method for comparing interventions, based on the published clinical trial literature, even where different outcome instruments are used. The “transfer to utility”, or TTU, allowed disparate trial outcome data to be translated into a common metric — a utility equivalent score — and the incorporation of mortality and costs to ascertain performance in terms of cost/QALY. This technique placed all treatments on a “level playing field”. The HsW model incorporating this technique should be less prone to the biases of more subjective priority-setting techniques, such as Program Budget Marginal Analysis, which allow “expert opinion” a greater role.6,7 The inclusion of the Advisory Panel in the process was found to be valuable for identification and interpretation of the literature, but not in development of the evidence base.
The quality of the advice resulting from the HsW model is dependent on the quality of clinical evidence. The modelling highlighted, in a systematic way, critical data gaps and future research priorities. Given the gaps in the data, outcomes and costs had to be modelled. This involved adopting assumed values and some limitation in scope, the latter reflecting the breadth of a priority-setting exercise relative to the research resources available. Most important of these limitations was the failure to conduct analyses at the subpopulation level, effectively assuming that mean incremental cost–utility ratio was a reasonable approximation across the relevant clinical population. Further, the scope of effects has been restricted to health benefits (defined by QALYs) and healthcare costs related to individuals with OA, a common (although not universal) assumption of cost-effectiveness analyses. In terms of technical simplifications, costs and benefits for a cohort should be based on the actual age distribution, rather than assuming a cohort of mean age consistent with the clinical trial information as we did in this study. Further, multivariate probabilistic sensitivity analysis is preferable to the use of univariate sensitivity analysis.
Despite these limitations, a number of conclusions can be developed from the HsW analysis, especially where clinical trial evidence is strong and the range of cost-utility estimates is narrow across plausible parameter values (Box 6). In drawing conclusions from the model it is presumed that cost-effectiveness is the sole or primary decision criterion. Given this premise, and the strong evidence of effectiveness and favourable cost–utility ratios for THR, TKR, intensive clinic-based exercise and strength training, and knee bracing, providing these services to all those for whom the treatment is clinically appropriate would represent an efficient use of healthcare resources. Subject to confirmatory evidence for efficacy and side-effect profile, topical capsaicin and oral glucosamine may also fit this category, with more modest effectiveness compensated by lower costs. The need for better targeting of COX-2 NSAIDs to subpopulations with greater capacity for net benefit is suggested by the wide range of cost–utility estimates (relative to placebo), and dominance by non-specific NSAIDs in many scenarios. Our analysis suggests that the resources released could be redirected to interventions that yield greater benefit per unit cost to reduce the overall disease burden of OA. Additional research into NSAIDs to highlight the performance of specific formulations and the impact on identifiable subpopulations is indicated. Independent of the HsW model, the literature suggests that the wide application of some treatments, such as arthroscopy for knee OA, may be a poor use of resources.29,45
It can be seen that economic analyses such as this can provide structured support to policy, and can complement clinicians’ judgement. How to achieve any agreed resource shifts and the optimal size of redistributions is properly the subject of a separate study. The translation of priority-setting recommendations into policy, and ultimately into health improvements for the population, will require the involvement of policymakers and clinical communities. The Bone and Joint Decade and its commitment to reducing the burden of disease from OA may contribute to this process.
1: Interventions for prevention and management of osteoarthritis (OA) that were included in the modelling
Primary prevention
Weight loss
Comprehensive media/community campaign;8,9 intensive primary care (GP and nurse) diet and behavioural weight loss intervention for a general overweight group;10 intensive primary care weight loss intervention for people with previous knee injury;10,11 surgery for obesity (eg, gastric banding)12
Patient management
Patient education
Lay-led program;13,14 GP/clinical nurse educator-led program15,16
Physical therapies
Specially fitted knee brace for person with OA of the knee17
Exercise/strength training
Home-based basic program;18 home-based intensive program;19 primary care clinic-based intensive program;20 out-patient-based intensive program21
Pharmacotherapies
Oral glucosamine sulfate;25 ASU (avocado/soybean unsaponifiables);26 topical capsaicin27
Surgery
Total knee replacement;28 total hip replacement;28 knee arthroscopy with lavage29
2: Relationship between observed assessment of quality of life utility values and utility values predicted using SF-36 subscales, based on a sample of 303 people with osteoarthritis
3: Conversion of SF-36 subscale scores to a utility score for osteoarthritis of the hip
|
|
Before surgery |
12 months after surgery |
||||||||
SF-36 subscale |
Regression weight |
SF-36 score* |
Adjustment† |
Utility score‡ |
SF-36 score* |
Adjustment† |
Utility score‡ |
||||
(Constant) |
– 0.1976 |
|
|
|
|
|
|
||||
Physical function |
0.4803 |
26.9 |
0.4661 |
0.224 |
66.6 |
0.7345 |
0.353 |
||||
Role physical |
– 0.0001 |
14.6 |
|
– 0.002 |
58.7 |
|
– 0.007 |
||||
Bodily pain |
0.2438 |
32.9 |
0.4562 |
0.111 |
72.8 |
0.7486 |
0.182 |
||||
General health |
0.0003 |
66.3 |
|
0.023 |
73.8 |
|
0.025 |
||||
Vitality |
0.0009 |
47.1 |
|
0.045 |
67.5 |
|
0.064 |
||||
Social function |
0.0018 |
52.5 |
|
0.092 |
88.6 |
|
0.155 |
||||
Role emotion |
– 0.0003 |
60.0 |
|
– 0.018 |
71.8 |
|
– 0.022 |
||||
Mental health |
0.0026 |
71.4 |
|
0.187 |
81.7 |
|
0.214 |
||||
Estimated utility value§ |
|
|
|
0.464 |
|
|
0.767 |
||||
Utility change |
|
|
|
|
|
|
0.304 |
||||
* Source: Bachmeier, March and Cross.28 † The physical function and bodily pain scales undergo an initial non-linear transformation. The physical function score of 26.9 is transformed by means of a quadratic adjustment: 0.108164 + (0.01595 × 26.9) – (0.000098 × 26.92) = 0.4663, which is multiplied by the regression weight of 0.4803 to obtain the utility component of 0.224. ‡ SF-36 subscale is multiplied by the subscale-specific regression weight. For example, the mental health score of 71.4 is multiplied by the regression weight of 0.0026 to obtain a “utility component” score of 0.187. § The utility component of each subscale is summed with the constant to obtain a total “utility equivalent” score. |
|||||||||||
4: Key assumptions underlying the modelling
Attribute and modality |
Key assumption |
||||||||||
Duration of benefits |
|
||||||||||
Primary prevention |
20 years |
||||||||||
Education/self-management |
2 years |
||||||||||
Exercise |
1 year |
||||||||||
Knee brace |
18 months to 3 years |
||||||||||
Total hip and knee replacement |
15 years, adjusted for all-cause mortality
Revision surgery, 12% or 20%, spread evenly over 15 years |
||||||||||
Non-steroidal anti-inflammatory drugs* |
12 months (treatment contiguous with benefit) |
||||||||||
Natural therapies |
12 months (treatment contiguous with benefit) |
||||||||||
Discount rate all modalities |
|
||||||||||
Downstream costs/cost savings |
Beyond 12 months, discounted at 5% per annum |
||||||||||
QALY gains or losses |
0 (undiscounted) 5% per annum |
||||||||||
Excess morbidity |
|
||||||||||
Non-steroidal anti-inflammatory drugs* |
Hospitalisation:
|
||||||||||
Excess mortality |
|
||||||||||
Non-steroidal anti-inflammatory drugs* |
Death rate:
Translation of deaths to life-years: mortality × 18.5 life-years (mean life expectancy, age 65 years) |
||||||||||
Total hip and knee replacement |
Death rate from surgery, 1/1000 |
||||||||||
Sensitivity analyses |
Discount rate of QALYs, 0 and 5% Clinical trial results: ± 1 SD (NSAIDs) or ± 2 SDs (other) |
||||||||||
NSAID = non-steroidal anti-inflammatory drug. QALY = quality-adjusted life-year. PUBs = perforations, peptic ulcerations, gastric bleeding. * Diclofenac, naproxen, celecoxib. |
|||||||||||
5: Key results of cost–utility analyses
Program |
Mean QALY gain per person* (zero discount rate) |
Mean program cost per person |
Cost/QALY† best estimate |
||||||||
Primary prevention |
|
|
|
||||||||
1. Comprehensive mass media program for weight loss |
|
$4 |
$20 000 |
||||||||
2. Intensive primary care weight loss program delivered by GP or dietitian for overweight or obese people |
0.09 |
$720 |
$11 000 |
||||||||
3. Intensive primary care weight loss program delivered by GP or dietitian for overweight or obese people with previous knee injury |
0.08 |
$720 |
$12 000 |
||||||||
4. Surgery for obese people |
1.05 |
$15 000 |
$20 000 |
||||||||
Management |
|
|
|
||||||||
Education |
|
|
|
||||||||
5. Lay-led group education |
0.072 |
$162 |
$2 400 to ∞ |
||||||||
6. Primary care: GP or clinical nurse educator plus phone support |
Equivocal |
$200–$400 |
‘ | ||||||||
Exercise/strength training |
|
|
|
||||||||
7. Home-based exercise — basic |
0.022 |
$400 |
$18 000 to ∞ |
||||||||
8. Home-based exercise — intensive |
0.100 |
$1 420 |
$15 000 |
||||||||
9. Clinic-based exercise — primary care |
0.091 |
$480 |
$5 000 |
||||||||
10. Clinic-based exercise — outpatients |
0.078 |
$590 |
$8 000 |
||||||||
Knee brace |
|
|
|
||||||||
11. Specially fitted knee brace |
0.12 to − 0.355 |
$1 300 |
$6 000 |
||||||||
Pharmacotherapies: prescription or over-the-counter medications |
|
|
|
||||||||
12. Non-specific NSAIDs (naproxen, diclofenac) |
+ 0.043‡ – 0.029 to 0.044§ |
Drug, $104/year; Morbidity, $70 |
$15 000 or ∞¶ |
||||||||
13. COX-2-specific NSAIDs (celecoxib) |
+ 0.043‡ – 0.029 to 0.044§ |
Drug, $391/year; Morbidity, $70 |
$33 000 or ∞¶ |
||||||||
Complementary medicines |
|
|
|
||||||||
14. Glucosamine sulfate |
0.052 |
$180 |
$3 000 |
||||||||
15. Avocado/soy unsaponifiables |
0.081 |
$333 |
$5 000 to ∞ |
||||||||
16. Topical capsaicin |
0.053 |
$236 |
$5 000 |
||||||||
Surgery |
|
|
|
||||||||
17. Total knee replacement |
+ 2.086‡ – 0.015§ |
$16 500 |
$8 000¶ or $11 000 |
||||||||
18. Total hip replacement |
+ 3.52‡ – 0.015§ |
$16 500 |
$5 000¶ or $7 000 |
||||||||
19. Knee arthroscopy with lavage |
Equivocal |
$3 500 |
∞ |
||||||||
* Relative to placebo, or to no intervention, for total hip and knee replacement surgery. † Rounded to nearest $1000; both costs and benefits discounted at 5% unless otherwise stated. ‡ Benefit from symptom control. § Mortality from cardiac and gastrointestinal side effects for NSAIDs or perioperative mortality for total hip replacement, total knee replacement, deducted from QALY gain. ¶ Zero discounting of future life-years lost from current deaths. QALY = quality-adjusted life-year. NSAIDs = non-steroidal anti-inflammatory drugs. ∞ = No evidence of benefit, possible negative benefit. |
|||||||||||
6: Resource implications for the prevention and management of osteoarthritis based on cost-utility analyses
Highly cost-effective programs (cost/QALY < $15 000) supported by strong evidence
Intensive clinic outpatient-based exercise/strength training programs
Total hip replacement surgery
Total knee replacement surgery
Policy implication: Programs/service level should meet all clinically indicated need.
Programs that appear highly cost-effective (cost/QALY < $10 000), but are based on limited evidence
Topical capsaicin
Glucosamine sulfate
Knee brace
Policy implication: Programs/services might warrant expansion to match clinical need. A program to collate/gather additional trial evidence should be supported. In the interim, greater access in response to clinical need appears appropriate.
Interventions which may be highly cost-effective (cost/QALY < $20 000), but based on indirect evidence
Primary prevention through weight loss in obese persons through comprehensive community-based media campaign, intensive primary care intervention, surgery for persons who are seriously obese (to determine impact on incidence of OA)
Policy implication: A research program should be supported, possibly through pilot implementation and/or well constructed observational studies.
Interventions for which cost-effectiveness is uncertain and highly sensitive to assumptions of the model (and the specific formulation)
Non-specific non-steroidal anti-inflammatory drugs
COX-2-specific non-steroidal anti-inflammatory drugs
Patient education — lay-led models
Policy implication: Explore assumptions of the model and cost-effectiveness in specific patient groups (and with particular formulations); redirect resources to patient groups where higher net benefit can be realised.
Interventions where efficacy is equivocal or not demonstrated
Patient education — professionally led models
Arthroscopy to manage knee OA
Policy implication: Services to be curtailed and resources redirected to areas with demonstrated effectiveness.
QALY = quality-adjusted life-year. OA = osteoarthritis.
- Leonie Segal1
- Susan E Day2
- Adam B Chapman3
- Richard H Osborne4
- 1 Health Economics Unit, Faculty of Business and Economics, Monash University, Melbourne, VIC.
- 2 Centre for Rheumatic Diseases, Department of Medicine, University of Melbourne, Melbourne, VIC.
We acknowledge funding from the Population Health Division, Department of Health and Ageing (DHA) and thank the Advisory Panel (in alphabetical order) — N Bellamy, University of Queensland; N Bogduk, University of Newcastle; P Brooks, University of Queensland; H Brown, DHA; R Buchbinder, Monash University; M Cohen, RMIT. J Feller, Austin Repatriation Medical Centre; M Galea, University of Melbourne; S Garner, DHA; B Harrison, DHA; J Kelly, Community Representative Victoria; S Kirsa, Austin and Repatriation Medical Centre; D Lewis, Monash University; L March, Royal North Shore Hospital; G McColl, University of Melbourne; H McNeil, Arthritis Foundation of Victoria; C Sindall, DHA; C Stone, Department of Human Services, Victoria; and P Woodley, DHA.
An Advisory Panel, including people from the DHA (see funding source, above), assisted by commenting on drafts and interpretation of the results. The authors controlled all intellectual decisions, and the views presented in this report are those of the researchers and not the DHA.
- 1. Australian Bureau of Statistics. National health survey. Canberra: Australian Bureau of Statistics, 1995. (Catalogue No. 4399.0.)
- 2. Segal L, Day S, Chapman A, et al. Priority setting in osteoarthritis. Report to the Department of Health and Ageing. Melbourne: Health Economics Unit, Monash University, 2004. In press.
- 3. Segal L, Robertson I. Diabetes integrated care trial mid-north coast, New South Wales. Research Report 21. Melbourne: Centre for Health Program Evaluation, 2001. Available at: http://chpe.buseco.monash.edu.au (accessed Jan 2004).
- 4. Carter R, Stone C, Vos T, et al. Trial of program budgeting and marginal analysis (PBMA) to assist cancer control planning in Australia (PBMA Series No 5). Research Report 19. Melbourne: Centre for Health Program Evaluation, 2000. Available at: http://chpe.buseco.monash.edu.au (accessed Jan 2004)..
- 5. Tengs TO, Adams ME, Pliskin JS, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Analysis 1995; 15: 360-390.
- 6. Segal L, Chen Y. Priority setting models for health. Working paper 119. Melbourne: Health Economics Unit, Monash University, 2001. Available at: http://chpe.buseco.monash.edu.au (accessed Jan 2004).
- 7. Coast J, Donovan J, Frankel S. Priority setting: the health care debate. New York: John Wiley, 1996.
- 8. Miles A. Using the mass-media to target obesity: an analysis of the characteristics and reported behaviour change of participants in the BBC’s “Fighting Fat, Fighting Fit” campaign. Health Educ Res 2001; 16: 357-372.
- 9. Wardle J, Rapoport L, Miles A, et al. Mass education for obesity prevention: the penetration of the BBC’s “Fighting Fat, Fighting Fit” campaign. Health Educ Res 2001; 16: 343-355.
- 10. Bowerman S, Bellman M, Saltsman P, et al. Implementation of a primary care physician network obesity management program. Obes Res 2001; 9 Suppl 4: 321S-325S.
- 11. Coggon D, Reading I, Croft P, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord 2001; 25: 622-627.
- 12. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS) — an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998; 22: 113-126.
- 13. Lorig K, Lubeck D, Kraines RG, et al. Outcomes of self-help education for patients with arthritis. Arthritis Rheum 1985; 28: 680-685.
- 14. Lorig K, Seleznick M, Lubeck D, et al. The beneficial outcomes of the arthritis self-management course are not adequately explained by behavior change. Arthritis Rheum 1989; 32: 91-95.
- 15. Mazzuca SA, Brandt KD, Katz BP, et al. Effects of self-care education on the health status of inner-city patients with osteoarthritis of the knee. Arthritis Rheum 1997; 40: 1466-1474.
- 16. Lord J, Victor C, Littlejohns P, et al. Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee. Health Technol Assess 1999; 3: 1-55.
- 17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am 1999; 81: 539-548.
- 18. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis 1999; 58: 15-19.
- 19. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol 2001; 28: 1655-1665.
- 20. van Baar ME, Dekker J, Oostendorp RA, et al. The effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a randomized clinical trial. J Rheumatol 1998; 25: 2432-2439.
- 21. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med 2000; 132: 173-181.
- 22. Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74: 1095-1105.
- 23. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum 2001; 44: 1587-1598.
- 24. Williams GW, Hubbard RC, Yu SS, et al. Comparison of once-daily and twice-daily administration of celecoxib for the treatment of OA of the knee. Clin Ther 2001; 23: 213-227.
- 25. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001; 357: 251-256.
- 26. Appelboom T, Schuermans J, Verbruggen G, et al. Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. A double blind, prospective, placebo-controlled study. Scand J Rheumatol 2001; 30: 242-247.
- 27. Altman R, Aven A, Holmburg C, et al. Capsaicin cream 0.025% as monotherapy for osteoarthritis: a double-blind study. Semin Arthritis Rheum 1994; 23: 25-33.
- 28. Bachmeier C, March L, Cross M. A comparison of outcomes in OA patients undergoing total hip and knee replacement. Osteoarth Cartilage 2001; 9: 137-146.
- 29. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum 2002; 46: 100-108.
- 30. Kind P. The EuroQol instrument: an index of health-related quality of life. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia: Lippincott–Raven, 1996.
- 31. Osborne RH, Hawthorne G, Lew EA, et al. Quality of life assessment in the community-dwelling elderly: validation of the Assessment of Quality of Life (AQoL) instrument and comparison with the SF-36. J Clin Epidemiol 2003; 56: 138-147.
- 32. Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999; 8: 209-224.
- 33. Ware J, Kosinski M, Keller S. SF-36 physical and mental health summary scales: a user’s manual. Boston: The Health Institute, New England Medical Centre, 1994.
- 34. Brazier J, Usherwood T, Harper R, et al. Deriving a preference-based single index from the UK SF-36 health survey. J Clin Epidemiol 1998; 51: 1115-1128.
- 35. Hawthorne G, Richardson J, Day N. A comparison of the Assessment of Quality of Life (AQoL) with four other generic utility instruments. Ann Med 2001; 33: 358-370.
- 36. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21: 271-292.
- 37. Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: Australian Institute of Health and Welfare, 1999. (AIHW cat no PHE 17.)
- 38. Bellamy N, Kaloni S, Pope J, et al. Quantitative rheumatology: a survey of outcome measurement procedures in routine rheumatology outpatient practice in Canada. J Rheumatol 1998; 25: 852-858.
- 39. Richardson J. Age weighting and time discounting: technical imperative versus social choice. In: Summary measures of population health. Geneva: World Health Organization, 2002: 663-676.
- 40. Department of Health. Policy appraisal and health: a guide from the Department of Health. London: Department of Health, 1996.
- 41. The Green Book, Appraisal and Evaluation in Central Government. London: HM Treasury, January 2003.
- 42. Department of Health and Ageing. Guidelines for the pharmaceutical industry on preparation of submissions to the Pharmaceutical Benefits Advisory Committee. Canberra: Commonwealth Department of Health and Ageing, 2002.
- 43. Crawford RW, Murray DW. Total hip replacement: indications for surgery and risk factors for failure. Ann Rheum Dis 1997; 56: 455-457.
- 44. Annual report of the Australian Orthopaedic Association. Available at: http://www.dmac.adelaide.edu.au/aoanjrr/publications.jsp (accessed Apr 2003).
- 45. Moseley JB Jr, Wray NP, Kuykendall D, et al. Arthroscopic treatment of OA of the knee: a prospective randomised placebo-controlled trial. Results of a pilot study. Am J Sports Med 1996; 24: 28-34.
- 46. Juni P, Rutjes AW, Dieppe PA. Are selective COX2 inhibitors superior to traditional non steroidal anti-inflammatory drugs? BMJ 2002; 324: 1287-1288.
- 47. Rindone JP, Hiller D, Collacott E, et al. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med 2000; 172: 91-94.
- 48. Muller-Fassbender H. Double-blind clinical trial of S-adenosylmethionine versus ibuprofen in the treatment of osteoarthritis. Am J Med 1987; 83: 81-83.
- 49. Cohen M, Wolfe R, Mai T, Lewis D. A randomized, double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J Rheumatol 2003; 30: 523-528.
- 50. Pavelka K, Gatterova J, Olejarova M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 2002; 162: 2113-2123.





Abstract
The comparison of disparate interventions for the prevention and management of osteoarthritis (OA) is limited by the quality and quantity of published efficacy studies and the use of disparate measures for reporting clinical trial outcomes.
The “transfer to utility” technique was used to translate published trial outcomes into a health-related quality-of-life (utility) scale, creating a common metric which supported comparisons between disparate interventions.
Total hip replacement (THR) and total knee replacement (TKR) surgery were the most effective treatments and also highly cost-effective, at estimated cost per quality-adjusted life-year (QALY) of $7500 for THR and $10 000 for TKR (best estimate).
Other apparently highly cost-effective interventions were exercise and strength training for knee OA (< $5000/QALY), knee bracing, and use of capsaicin or glucosamine sulfate (< $10 000/QALY).
The cost per QALY estimates of non-specific and COX-2 inhibitor non-steroidal anti-inflammatory drugs were affected by treatment-related deaths and highly sensitive to the discounting of life-years lost.
OA interventions that have been shown to be ineffective (eg, arthroscopy) are targets for redistribution of healthcare resources.
OA interventions which lack efficacy studies (eg, prevention programs) require further research to assist priority setting.
The application of the Health-sector Wide model to OA demonstrates its role as an evidence-based model that can be successfully applied to identify marginal interventions — those to be expanded and contracted to reduce the expected burden of disease, within current healthcare resources.