Osteoporosis, one of the more common diseases in ageing populations, is a major public health problem because of its age-associated exponential increase in prevalence, and its serious consequences in terms of mortality, morbidity and economic costs. Osteoporotic fractures, commonly of the hip, spine, humerus, forearm and wrist, are typically sustained with little or no antecedent trauma. Vertebral fractures, which can vary from mild wedge compressions to complete crush fractures, are clinically silent in two-thirds of women. However, they are associated with considerable morbidity, increased risk of mortality, and indicate a greatly increased risk of further fractures, including hip fractures. The combined lifetime risk for hip, forearm and vertebral fractures coming to clinical attention is around 40%, equivalent to the risk for cardiovascular disease.1 In white women, the lifetime risk of hip fracture, the most serious consequence of osteoporosis, is 1 in 6, compared with a 1-in-9 risk of a diagnosis of breast cancer.2 In Australia alone, direct costs attributable to osteoporotic fractures were estimated at $700 million in 1994.3 More recent estimates, taking into account all types of fractures and all types of costs, both direct and indirect, have been estimated at about $7 billion annually.4 Death is relatively common in the months immediately after a hip fracture.5,6 Moreover, all major osteoporotic fractures are associated with a twofold increase in age-adjusted mortality in women and a threefold increase in men.6 Hip fracture survivors have an increased risk of dependence, with 50% requiring help with daily living activities and 15%–25% entering long-term care.7,8
Fracture risk is a function of trauma sustained (eg, in falls) and bone strength (which depends on both the quantity of bone and its architecture). Bone mass, assessed by bone mineral density (BMD), is a good predictor of fracture risk, with each standard deviation (SD) decrease in BMD being associated with at least a twofold increase in the risk of fracture.8-10 The relationship between BMD and fracture applies throughout the skeleton, with some site specificity (ie, hip fracture risk is more related to BMD measurements at the hip than at the lumbar spine or forearm). The strength of the relationship between fracture risk and BMD is stronger than that between stroke and blood pressure or between cholesterol level and myocardial infarct. Although low-trauma fracture is the hallmark of osteoporosis, it is usually classified in terms of BMD values. In 1994, an expert panel of the World Health Organization (WHO) proposed that osteoporosis be defined by BMD values relative to those of young adult females, with those more than 2.5 SDs below being classed as osteoporosis, and those between 1 and 2.5 SDs below being classified as osteopenia.11 By these criteria, in Australia, about 11% of men and 27% of women aged 60 or more years are osteoporotic, and another 42% of men and 51% of women are osteopenic.12 Of course, BMD is a continuous variable and the lower the BMD, the greater the relative risk of fracture. Absolute risk of fracture depends on BMD, bone shape and other poorly defined characteristics of bone “quality”, and the likelihood of trauma. Quantitative ultrasound measurements, such as broadband ultrasound attenuation (BUA) and speed of sound (SOS), have been shown to identify some people at increased risk of fracture in both cross-sectional and longitudinal studies.13-16 However, these people are not the same as those identified by BMD measurement, and they have not been shown to benefit in interventional clinical trials of fracture risk.
Common risk factors for osteoporosis include familial predisposition, lifestyle, hormonal, medications and illnesses and falls (see Box 1). Inherited factors play an important role in osteoporosis risk. In studies of twins, 70%–80% of differences in BMD (measured at the lumbar spine and femoral neck) between individuals is attributable to genetic factors.17-19 Various studies have found significant inherited components of fracture risk (eg, 25% of liability for Colles’ fracture, a twofold hip fracture risk with a maternal hip fracture, a threefold risk of hip and other fractures with a paternal wrist fracture).20,21 Certain key genes have been reported as contributors to this inherited risk, with variants of each being associated with a doubled risk of osteoporotic fracture (eg, the vitamin D receptor, collagen Ia1, the Wnt-Frizzled pathway, and bone morphogenetic protein 2).
Hormonal and reproductive factors influence osteoporosis risk, as do medications and certain underlying diseases. Oestrogen deficiency, from natural or surgically induced menopause, or resulting from excessive exercise or undernutrition, accelerates bone loss. Women with late menarche and/or early menopause, and thus a shorter exposure to normal reproductive hormones, may be at increased risk.22-25 Many of these factors are not amenable to intervention; hormone replacement therapy is the exception, but its use has decreased greatly following recent studies relating oestrogen use to (modestly) increased risk of breast cancer and cardiovascular and cerebrovascular outcomes.
People with a history of prior fracture are at significantly increased risk of subsequent fracture. For instance, women with pre-existing vertebral fractures have a risk of subsequent vertebral fractures about 4 times greater than those without prior fractures, and the risk increases with the number of prior vertebral fractures.26
Nutrition and lifestyle also affect the skeleton, although this may be more relevant at the extremes of variables such as nutrition and exercise than within normal ranges. Thinness is an important risk factor for low BMD. Indeed, of all anthropometric factors, body weight is the strongest predictor of BMD. The positive relationship between increased weight and increased BMD may be partly attributable to increased mechanical forces on the bone,27 but neural control factors may also play a part.28 An adequate dietary calcium intake and a physically active lifestyle in later decades of life could translate into a reduction in the risk of osteoporosis. For example, women in the top third for both quadriceps muscle strength and dietary calcium intake had 15% higher BMD than those in the lowest third. Among people in the lowest third for body mass index (< 23–24 kg/m2), quadriceps strength and dietary calcium intake (< 465 mg/day), about two-thirds of women and almost half of men had osteoporosis by WHO BMD criteria.29
Smoking is associated with lower BMD and increased fracture risk in postmenopausal women30-32 and in men.31 However, a positive effect in bone, as assessed by BMD, is associated with modest alcohol intake (ie, about 3–4 units per week in premenopausal and postmenopausal women31,33). Some medications may modify osteoporosis risk, including, particularly, excessive thyroxine replacement therapy and oral glucocorticoid use; the latter has been associated with increased risk of spine and forearm fractures.34
Change in BMD is the result of the bone remodelling process (or bone turnover), in which microscopic amounts of bone tissue are removed (bone resorption) and then replaced with new tissue (bone formation).35 In adulthood, with an increased rate of bone turnover, the rate of bone resorption is greater than the rate of bone formation, resulting in net bone loss both in trabecular and cortical bone. At present, most pharmacological therapies for treating osteoporosis aim to inhibit this excessive bone resorption.36 These agents (see Box 2), which include bisphosphonates and raloxifene, as well as oestrogen replacement therapy, reduce the rate of bone turnover (particularly bone resorption), reduce bone loss and increase BMD measurements. In randomised studies of varying quality, these agents have been shown to reduce fracture risk. In postmenopausal women with low BMD who have or have not had previous vertebral fracture, hormone replacement therapy (HRT),37-39 raloxifene,40-42 and bisphosphonates such as alendronate43-45 and risedronate46,47 have each been shown to increase BMD and reduce fracture risk by between 30% and 60%. Parathyroid hormone (PTH [1-34]), which stimulates bone formation, has also been shown to increase BMD, improve bone structure, and decrease the risk of vertebral and non-vertebral fractures.48 The efficacy of combination therapies has also been investigated,49,50 but data are scarce and study sizes too small to determine whether fracture risk would be significantly reduced compared with monotherapy.
Vitamin D3 and calcium have been shown in some (but not all) randomised clinical trials to reduce hip fractures by 43% and other non-vertebral fractures by 32% in ambulatory women living in nursing homes, and to reduce non-vertebral fractures in men and women aged 65 years or older living in the community.51-53 Vitamin D treatment is logical in institutionalised or housebound elderly people, in whom vitamin D deficiency is common. Hip protectors have been reported to reduce hip fractures in some, but not all, clinical trials.54,55 Also, compliance with wearing hip protectors can be a problem, primarily because of discomfort.
It is generally agreed that individuals with low BMD, and particularly those with a history of fracture (with osteoporosis or osteopenia), should be considered for treatment. However, recent studies have shown that these high-risk individuals are not being diagnosed or treated. Among hospitalised women aged 60 or older with spine radiographs showing severe vertebral deformities, only 17% had mention of the fracture in their medical records or discharge summary.56 A study of women aged 55 years or more with wrist fractures in a managed-care setting reported that 23% had been started on some form of osteoporosis-specific therapy, and less than 3% had had a BMD scan.57 The situation for hip fracture is more disturbing. In a study of 502 hospitalised hip-fracture patients, only 14% had BMD scans, 13% received calcium and/or vitamin D, and only 18% received HRT, calcitonin, or bisphosphonates.58 Other studies have reported that only 5% of patients with recent hip fractures left the hospital with a new medication prescribed for reducing the risk of subsequent fractures.59,60 In Australia, the situation is no better. In a recent survey of more than 88 000 women attending 927 primary care physicians, of those who reported a fracture postmenopause less than 20% were on any specific antiosteoporotic therapy (personal data). Thus, despite both the magnitude of the problem and the introduction of osteoporosis treatment guidelines, most high-risk individuals (possibly 80%) are still not identified, and thus not treated. It may be reasonable to infer that many otherwise preventable fractures are occurring daily in Australia, as well as around the world.
Osteoporosis is a complex and costly disease. Like many other multifactorial diseases, its occurrence is determined by an array of environmental factors, by genetic susceptibility, and likely by their interactions. From the population perspective, osteoporotic fractures may be largely preventable, as environmental factors are open to intervention, and effective pharmacological agents are available. From both clinical and economic perspectives, aggressive measures to detect osteoporosis at earlier stages may be warranted. Yet, at present, the great majority of individuals at high-risk, who have already had at least one osteoporotic fracture, are neither identified nor treated. This reality calls for major steps, including operational research, to identify and remove barriers to more effective prevention of osteoporosis. Medical fracture clinics in all major and teaching hospitals could ensure that at least all individuals who have had possible osteoporotic fractures are suitably investigated and treated to reduce their risk of subsequent fractures. This first step might also help raise awareness and increase the likelihood of earlier intervention before the first fracture occurs in other individuals.
1: Some common risk factors for osteoporotic fractures
Genetic factors
Family history of fracture
Nutrition and lifestyle factors
Inadequate dietary calcium intake
Sedentary lifestyle or physical inactivity
Smoking
Excessive alcohol intake
Hormonal and reproductive factors
Early or surgically induced menopause
Short duration of reproduction lifetime (ie, late menarche and/or early menopause)
Gonadotropin-releasing-hormone agonist
Anorexia nervosa
Low testosterone levels in men
Vitamin D deficiency
Low body weight
Hyperthyroidism
Medications
Corticosteroids
Diuretics (positive effect)
Comorbidity
Malabsorption with intestinal disease (eg, coeliac disease)
Rheumatoid arthritis
Prolonged bed rest
Fall-related factors
Poor strength in quadriceps
Postural instability
Visual impairment
2: Therapeutic options for osteoporosis
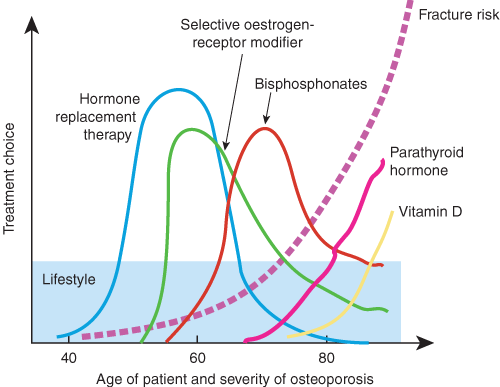
Adequate calcium intake and physical activity, as well as avoidance of smoking and excessive alcohol intake, underpin prevention and treatment of osteoporosis from childhood to old age. Choices of other specific treatments relate to an individual’s age and severity of osteoporosis (dashed line), which generally worsens with advancing age.
- Tuan V Nguyen1
- Jacqueline R Center2
- John A Eisman3
- Bone and Mineral Research Program, Garvan Institute of Medical Research, Darlinghurst, NSW.
None identified.
- 1. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002; 359: 1929-1936.
- 2. Cummings SR, Melton LJ III. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359: 1761-1767.
- 3. Randell A, Sambrook PN, Nguyen TV, et al. Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos Int 1995; 6: 427-437.
- 4. The burden of brittle bones — costing osteoporosis in Australia. Presented for Osteoporosis Australia by Access Economics Pty Ltd. Canberra: Access Economics, September 2001.
- 5. Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999; 353: 878-882.Hasserius R, Karlsson MK, Nilsson BE, et al. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003; 14: 61-68.
- 6. Randell AR, Nguyen TV, Sambrook PN, Eisman JA. Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int 2000; 11: 460-466.
- 7. Scaf-Klomp W, van Sonderen E, Sanderman R, et al. Recovery of physical function after limb injuries in independent older people living at home. Age Ageing 2001; 30: 213-219.
- 8. Nguyen TV, Sambrook PN, Kelly PJ, et al. Prediction of osteoporotic fractures by postural instability and bone density. BMJ 1993; 307: 1111-1115.
- 9. Melton LJ, Atkinson EJ, O’Fallon WM, et al. Longterm fracture risk prediction by bone mineral density assessed at different skeletal sites. J Bone Miner Res 1993; 8: 1227-1233.
- 10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312: 1254-1259.
- 11. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368-381.
- 12. Nguyen TV, Eisman JA. Risk factors for low bone mass in elderly men. In: Orwoll ES, editor. Osteoporosis in men. San Diego: Academic Press, 1999: 335-362.
- 13. Schott AM, Weill-Engerer S, Hans D, et al. Ulstrasound discriminates patients with hip fracture equally well as dual energy x-ray absorptiometry and independent of bone mineral density. J Bone Miner Res 1995; 10: 243-249.
- 14. Glüer CC, Cummings SR, Bauer DC, et al. Osteoporosis: association of recent fractures with quantitative ultrasound findings. Radiology 1996; 199: 725-732.
- 15. Bauer DC, Gluer CC, Cauley JA, et al. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1997; 157: 629-634.
- 16. Hans D, Dargent-Molina P, Schott AM, et al. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 1996; 348: 511-514.
- 17. Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass and fat mass: same genes or same environments. Am J Epidemiol 1998; 147: 3-16.
- 18. Howard GM, Nguyen TV, Harris M, et al. Genetic and environmental contributions to the association between quantitative ultrasound and bone mineral density measurements: a twin study. J Bone Miner Res 1998; 13: 1318-1327.
- 19. Flicker L, Hopper JL, Rodgers L, et al. Bone density determinants in elderly women: a twin study. J Bone Miner Res 1995; 10: 1607-1613.
- 20. Deng HW, Chen WM, Recker S, et al. Genetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fracture. J Bone Miner Res 2000; 15: 1243-1252.
- 21. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med 1995; 332: 767-773.
- 22. Riggs BL, Melton LJ III. Involutional osteoporosis. N Engl J Med 1986; 314: 1676-1686.
- 23. Khosla S, Bilezikian JP. The role of estrogens in men and androgens in women. Endocrinol Metab Clin North Am 2003; 32: 195-218.
- 24. Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002; 359: 1841-1850.
- 25. Nguyen TV, Jones G, Sambrook PN, et al. The effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab 1994; 9: 2709-2714.
- 26. Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15: 721-739.
- 27. Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: The Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 1998; 13: 1458-1467.
- 28. Takeda S, Elefteriou F, Karsenty G. Common endocrine control of body weight, reproduction, and bone mass. Ann Rev Nutr 2003; 23: 403-411.
- 29. Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity and body mass index. J Bone Miner Res 2000; 15: 322-331.
- 30. Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 2001; 68: 259-270.
- 31. Nguyen TV, Kelly PJ, Sambrook PN, et al. Lifestyle factors and bone density in the elderly: implications for osteoporosis prevention. J Bone Miner Res 1994; 9: 1339-1346.
- 32. Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 1997; 315: 841-846.
- 33. Hermann AP, Brot C, Gram J, et al. Premenopausal smoking and bone density in 2015 perimenopausal women. J Bone Miner Res 2000; 15: 780-787.
- 34. Adinoff AD, Hollister MD. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med 1983; 309: 265-268.
- 35. Miller PD, Baran DT, Bilezikian JP, et al. Practical clinical application of biochemical markers of bone turnover: consensus of an expert panel. J Clin Densitom 1999; 2: 323-342.
- 36. Delmas PD. Treatment of postmenopausal osteoporosis. Lancet 2002; 359: 2018-2026.
- 37. Cauley JA, Seeley DG, Ensrud K, et al. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med 1995; 122: 17-23.
- 38. Torgerson DJ, Bell-Syer SEM. A meta-analysis of hormone replacement therapy for fracture prevention. JAMA 2001; 286: 2096-2097.
- 39. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002; 288: 321-333.
- 40. Johnston CC Jr, Bjarnason NH, Cohen FJ, et al. Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women — three-year data from 2 double-blind, randomized, placebo-controlled trials. Arch Intern Med 2000; 160: 3444-3450.
- 41. Prestwood KM, Gunness M, Muchmore DB, et al. A comparison of the effects of raloxifene and estrogen on bone in postmenopausal women. J Clin Endocrinol Metab 2000; 85: 2197-2202.
- 42. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) investigators. JAMA 1999; 282: 637-645.
- 43. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial. Lancet 1996; 348: 1535-1541.
- 44. Karpf DB, Shapiro DR, Seeman E, et al. Prevention of nonvertebral fractures by alendronate: a meta-analysis. JAMA 1997; 227: 1159-1164.
- 45. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. J Clin Endocrinol Metab 2000; 85: 4118-4124.
- 46. Reginster J, Minne HW, Sorensen OH, et al. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000; 11: 83-91.
- 47. Harris ST, Watts NB, Genant HK, et al, for the Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. JAMA 1999; 282: 1344-1352.
- 48. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434-1441.
- 49. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med 1999; 130: 897-904.
- 50. Lindsay R, Cosman F, Lobo RA, et al. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomised, controlled clinical trial. J Clin Endocrinol Metab 1999; 84: 3076-3081.
- 51. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fracture in elderly women. N Engl J Med 1992; 327: 1637-1642.
- 52. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337: 670-676.
- 53. Gillespie WJ, Avenell A, Henry DA, et al. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis (Cochrane Review). In: The Cochrane Library, Issue 4, 2003. Chichester, UK: John Wiley & Sons, Ltd.
- 54. Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly. Cochrane Database of Systematic Reviews. Cochrane Library 2001, Issue 4. Available at: www.update-software.com/abstracts/ab001255.htm (accessed Dec 2003).
- 55. van Schoor NM, Smit JH, Twisk JW, et al. Prevention of hip fractures by external hip protectors: a randomized controlled trial. JAMA 2003; 289: 1957-1962.
- 56. Gehlbach SH, Fournier M, Bigelow C. Recognition of osteoporosis by primary care physicians. Am J Public Health 2002; 92: 271-273.
- 57. Freedman KB, Kaplan FS, Bilker WB, et al. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am 2000; 82-A: 1063-1070.
- 58. Harrington JT, Broy SB, Derosa AM, et al. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum 2002; 47: 651-654.
- 59. Bauer DC. Osteoporotic fractures: ignorance is bliss? Am J Med 2000; 109: 338-339.
- 60. Kamel HK, Hussain MS, Tariq S, et al. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000 Sep; 109: 326-328.





Abstract
Osteoporosis is: