Thyroid dysfunction is common. In the United States, hypothyroidism is present in 4.6% of the population (clinical, 0.3%; and subclinical, 4.3%) and hyperthyroidism in 1.3% (clinical, 0.5%; and subclinical, 0.7%).1 A long-term study in the United Kingdom found the incidence of hyperthyroidism was 0.8 per 1000 women annually, and hypothyroidism was 3.5 per 1000 women annually.2
Thyroid function testing: Measurement of serum thyroid stimulating hormone (TSH), using second- or third-generation assays, is a sensitive index of primary thyroid disease.3 Assay of free thyroxine (T4) and triiodothyronine (T3) reliably eliminates the difficulties in interpretation of total thyroid hormone levels caused by the common variations in serum thyroid hormone-binding protein, best exemplified by the oestrogen-induced rise in thyronine-binding globulin which increases total thyroid hormone levels. However, free hormone assays are still subject to in-vitro and in-vivo artefacts in severe non-thyroidal illness, severe disturbances of binding proteins, and heparin therapy.4 Thus, if clinical assessment is not concordant with thyroid function results, additional tests and specialist opinion may be necessary. Interpretation of thyroid function results is summarised in Box 1.
Antibody testing: The most common cause of thyroid dysfunction in Australia is autoimmunity. The most sensitive in-vitro index for this is measurement of thyroid peroxidase antibody level,1 but this can occasionally give false negative results (eg, in juvenile autoimmune thyroiditis). In primary hypothyroidism, a raised level of thyroid peroxidase antibody is evidence for autoimmune chronic lymphocytic thyroiditis.
In hyperthyroidism, the role of routine antibody testing is less clear. Diagnosis of Graves disease is usually possible clinically, and measurement of thyroid peroxidase antibody or TSH-receptor antibody may not contribute to diagnosis or management. However, measurement of TSH-receptor antibody has a role when the cause of hyperthyroidism is obscure, in assessing the risk of neonatal hyperthyroidism in pregnant women with a history of Graves disease, and in assessing the risk of relapse after a course of antithyroid drugs in Graves disease.
Radionuclide scanning: Radionuclide thyroid scanning is not routinely necessary for diagnosing Graves disease or toxic multinodular goitre, but is useful when the cause of hyperthyroidism is not apparent (eg, when no goitre is palpable, when neck pain or tenderness suggests subacute thyroiditis, or when a solitary “hot” nodule is suspected).
The terms hyperthyroidism and thyrotoxicosis are used interchangeably, irrespective of whether the disorder is caused by endogenous overproduction or excessive ingestion of thyroid hormones. Causes of hyperthyroidism are shown in Box 2. Most common in Australia are Graves disease and toxic multinodular goitre. Management of hyperthyroidism depends on the cause (particularly whether it is self-limiting, as in subacute thyroiditis), the size and nature of the goitre, intercurrent illnesses or medication and, especially in Graves disease, patient preference.
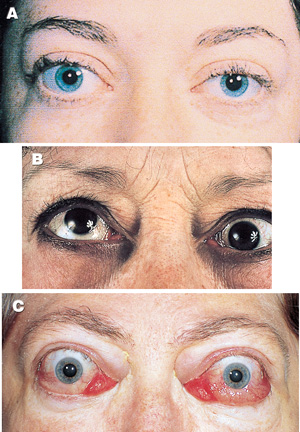
Graves disease is caused by stimulation of the thyroid by antibodies which bind to TSH receptors and mimic the effect of prolonged TSH stimulation. These TSH-receptor antibodies result from abnormal immunoregulation permitting generation and expansion of clone(s) of antibody-producing cells in genetically predisposed individuals with specific HLA-D subtypes.5 Spontaneous exacerbations and remissions of Graves disease can occur. The environmental triggers are still not well characterised, but postpartum exacerbation is common, and such a history should be sought routinely when Graves disease is diagnosed. Excess iodine can precipitate active Graves disease by providing more substrate for hormone synthesis and possibly also by disturbing immune function. Persistence or recurrence of Graves disease is more likely when there is a previous history of recurrent disease, in the presence of a large goitre, when T3 excess persists despite control of T4 with thionamide therapy, and when TSH-receptor antibody persists during thionamide therapy. In addition to hyperthyroidism, other autoimmune manifestations of Graves disease are Graves ophthalmopathy (Box 3) and Graves dermopathy (pretibial myxoedema). Autoimmune thyroid disease is associated with some other, less common, autoimmune diseases, including pernicious anaemia and Addison’s disease.
The three modalities of therapy for Graves disease are:
blocking synthesis of thyroid hormone with antithyroid drugs;
subtotal or “near-total” thyroidectomy; and
destruction of the thyroid by radioactive iodine (radio-iodine ablation).
While each modality is satisfactory in rendering the patient euthyroid,6 none is ideal, as all have a risk of adverse effects and none but total thyroidectomy eliminates the risk of recurrence. Although total thyroidectomy virtually eliminates this risk, it is at the expense of a certain requirement for thyroid hormone replacement. Selecting treatment for an individual depends on many factors, not least being patient choice and physician bias. In the United States, radioiodine is the preferred primary modality, but, in Europe and Australia, antithyroid drug therapy is preferred for patients with a first episode of Graves hyperthyroidism, ahead of radioiodine and, lastly, surgery.7
Antithyroid drugs: Most patients with Graves disease require short-term (several months) treatment with an antithyroid drug (thionamide) before consideration of longer-term or definitive therapy. Prolonged thionamide therapy (12–18 months in a first episode8) has the advantage of avoiding surgery with its inherent risks and destruction of the thyroid by radioiodine, and seems to give the best chance of sustained remission. Nonetheless, the risk of relapse is greater than 50%.
Thionamide dose must be individualised, depending on the initial severity of disease and response, but an initial divided dose of 10–30 mg daily of carbimazole is usually satisfactory. Response should be assessed after 2–4 weeks and periodically thereafter, with a minimum eventual frequency of every third month. Initial high doses should be progressively reduced to once-daily maintenance doses of 2.5–10 mg/day (Box 4).
Occasionally, in very active Graves disease, thionamide therapy can lower serum free T4 level below normal, while free T3 level remains raised, and the patient remains hyperthyroid. Thionamide dose should thus not be reduced on the basis of serum free T4 level alone. Clinical assessment remains paramount, guided by full thyroid function testing.
Combined thionamide and thyroxine therapy (block–replace regimen) is useful for patients with unstable hyperthyroidism, in whom small variations in thionamide dose cause major fluctuations in thyroid function, but does not increase the likelihood of long-term remission.9
Beta-blocker drugs are useful adjuncts for rapid symptomatic relief in hyperthyroidism. Standard doses reduce heart rate, sweating and tremor, but do not influence hypermetabolism or hormone levels. Non-selective β-blockers have generally been preferred for their better effect on tremor.
All patients should be warned about the rare but serious complication of thionamide therapy, agranulocytosis, with instructions to suspend therapy while obtaining a white cell count if fever, sore throat, or other sepsis develops. We do not recommend routine blood testing for this side effect, as it is rare and of abrupt onset.
Radioiodine ablation: Ablative therapy with radioiodine is recommended for patients with recurrent hyperthyroidism or hyperthyroidism that persists after a prolonged course of antithyroid drugs. Reassuringly, several large, long-term studies have shown no increased risk of thyroid cancer, leukaemia, other malignancies, reproductive abnormalities or congenital abnormalities in the offspring of treated patients.10 It is thus the default option for definitive therapy in adolescents and adults. More caution is recommended in children because of the known greater risk of inducing thyroid nodules and carcinoma from external irradiation and other radionuclide exposure in childhood.
Radioiodine therapy does not achieve euthyroidism immediately, necessitating low-dose thionamide therapy for several months in many patients. Occasionally, early (usually transient) hypothyroidism occurs, with low serum free T4 levels without TSH elevation, as TSH is often suppressed for weeks to months after hyperthyroidism.
The likelihood of control of hyperthyroidism after a single radioiodine treatment varies with the dose, continuing immunological stimulus of the thyroid, and thyroid responsiveness to the radioiodine. Overall, control is achieved initially in about 75% of cases. Eventual control through repeated doses, given after a minimum of six months, is virtually assured, but often at the expense of permanent hypothyroidism. The probability of developing hypothyroidism increases with the dose of radioiodine and the passage of time, so that long-term follow-up and patient education is required in all cases.
Thyroidectomy: Thyroidectomy is generally undertaken only after euthyroidism has been attained with thionamide therapy. The high rate of relapse after subtotal thyroidectomy has led many surgeons to recommend complete thyroidectomy (termed total or near-total thyroidectomy), but this inevitably necessitates permanent thyroid hormone replacement therapy. Thyroidectomy offers rapid control without radiation exposure and reliably removes a substantial goitre. It becomes the therapeutic choice when malignancy is suspected or a large goitre is causing local compression. As with all surgery, the individual skill of the surgeon is paramount, and there should be no role for the occasional exponent.
Toxic multinodular goitre is almost always preceded by long-standing multinodular goitre and probably, in most patients, by episodes of subclinical hyperthyroidism. Hyperthyroidism is often precipitated by exposure to excessive iodine from adventitious sources, such as medications and radiocontrast media used in imaging procedures (see list under Iodine-induced hyperthyroidism).11 The goitre may compress or obstruct the trachea. Plain radiography of the trachea may reveal this, but computed tomography is better, provided no contrast is administered. Magnetic resonance imaging best demonstrates the extent of the goitre but is not directly available to the general practitioner. Oesophageal symptoms occasionally require assessment by barium swallow. Venous obstruction, which is more common than oesophageal obstruction, is assessed clinically (Box 5).
The initial choice of therapy for toxic multinodular goitre is a thionamide to render the patient euthyroid. Spontaneous remission is not part of the natural history of this condition and, unless there has been a specific precipitating episode of iodine exposure, ablative therapy is indicated. In the absence of obstruction, radioiodine is the treatment of choice. Higher doses, and sometimes multiple treatments, may be necessary for a satisfactory outcome. If obstruction is present then thyroidectomy is indicated. Total thyroidectomy also obviates the risk of regrowth of the goitre.
A single hyperfunctioning nodule, usually an adenoma, is an uncommon cause of hyperthyroidism. If a single nodule is detected on clinical or ultrasound examination in a hyperthyroid patient, the diagnosis should be confirmed by a radionuclide scan. Radioiodine is the preferred treatment after control of thyroid function by a short course of thionamide. The risk of radiation-induced hypothyroidism is small in this condition, in contrast to Graves hyperthyroidism, as the hyperfunctioning nodule suppresses the rest of the thyroid.12 Lobectomy is still an acceptable choice.
Iodine can induce hyperthyroidism in patients with an underlying autonomously functioning thyroid gland (caused, for example, by a nodule, multinodular goitre or Graves disease). It is characterised by suppressed serum TSH level with normal circulating thyroid hormone levels, and is most commonly found in patients with longstanding multinodular goitre.13 A history should be sought of recent exposure to iodine from radiocontrast media and pharmaceuticals such as amiodarone, herbal and vitamin preparations, kelp and Cellasene (a preparation marketed as a treatment for cellulite).
As radiocontrast media are clear precipitants, computed tomography should be performed without contrast in the investigation of euthyroid goitre and undiagnosed neck mass.
The antiarrhythmic drug amiodarone has a 37% iodine content, with high tissue penetration and a half-life of months. The elemental iodine load of about 9 mg per day from a 200 mg tablet can precipitate hyperthyroidism if there is pre-existing goitre (type I amiodarone-induced hyperthyroidism), while the drug itself causes thyroiditis in 5%–10% of users, typically after about 2 years of therapy (type II amiodarone-induced hyperthyroidism, the most common form in Australia14). Although type I may respond to high-dose thionamide therapy, and type II to glucocorticoid therapy, these conditions can be severe and treatment-resistant, and thyroidectomy may be needed.
“Subclinical” hyperthyroidism is a poor term for this condition, as the definition is biochemical rather than clinical, and clinical consequences are possible. It is characterised by chronically suppressed TSH levels but normal serum free T4 and T3 levels. The condition must be distinguished from transient TSH suppression in acute illness or after drug therapy (eg, high-dose glucocorticoids). It occurs commonly with multinodular goitre, a hyperfunctioning single thyroid nodule and thyroxine overreplacement. Subclinical hyperthyroidism is associated with an increased risk of atrial arrhythmia in those aged over 60 years15 and loss of bone mineral density in postmenopausal women.16 Antithyroid therapy should be considered in patients with subclinical hyperthyroidism at increased risk of complications, and reduction of thyroxine dose in those taking replacement therapy. Subclinical hyperthyroidism is intentionally produced by TSH-suppressive thyroxine therapy for differentiated thyroid cancer, but it is unnecessary to continue this therapy lifelong if likely eradication of carcinoma has been demonstrated.
This is the deliberate, usually surreptitious, excessive ingestion of thyroid hormone.17 It is often part of a wider psychiatric condition and may be refractory to medical advice. It may be severe, with weight loss, weakness, cardiac tachyarrhythmia and failure. When due to thyroxine ingestion, serum free T4 level is elevated disproportionately to T3 level, but, when due to liothyronine (T3) ingestion, serum free T4 level is suppressed. Thyroid radionuclide uptake and serum thyroglobulin are suppressed. No treatment strategy is known to be particularly effective, but non-judgemental explanation of the medical consequences and gradual dose reduction with psychological supportive therapy, if accepted, is recommended.
TSH-secreting pituitary tumours constitute less than 1% of all pituitary tumours. Thyroid stimulation results in hyperthyroidism with a diffuse goitre, clinically similar to Graves disease. The presence of high serum free T4 and T3 levels with an unsuppressed serum TSH level suggests the diagnosis.18 Other clinical conditions that need to be distinguished include the partial (pituitary) resistance form of inherited thyroid hormone resistance syndrome,19 and anomalous laboratory TSH results caused by heterophil antibodies (common antibodies that interfere with assays using mouse monoclonal antibodies; they are either generated directly against mouse antigens after exposure to mice or cross-react with these antigens).
The most common cause of hypothyroidism is primary failure of the thyroid gland. While secondary hypothyroidism from pituitary or hypothalamic dysfunction is rare, it is vital to identify the site of dysfunction at the outset. As serum TSH levels rise logarithmically in response to declining thyroid hormone levels, the distinction between primary and secondary hypothyroidism is usually obvious. The situation can, however, be confounded by impaired pituitary response in concomitant severe non-thyroidal illness, occasionally in ageing, and in profound prolonged hypothyroidism. A modest TSH elevation (up to 15–20 mU/L) in the recovery phase from the severe sick euthyroid state (eg, after acute trauma, burns or infection), or on cessation of dopamine infusion,20 can cause diagnostic confusion.
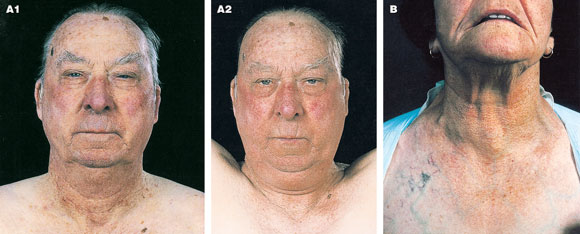
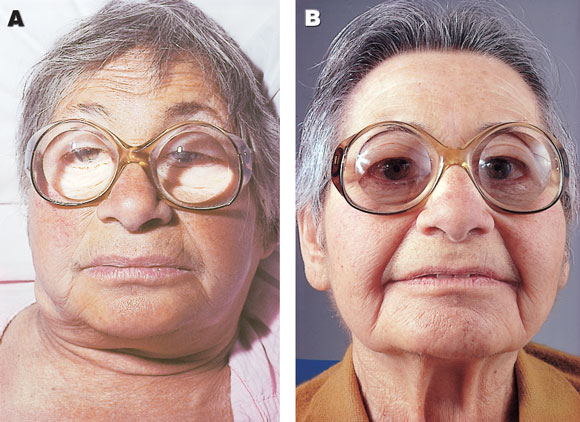
Hypothyroidism covers a wide spectrum of clinical and biochemical disease, from clinically inapparent disease to myxoedema coma (Box 6). The causes of hypothyroidism are shown in Box 7.
In Australia, the most common cause of hypothyroidism is autoimmune chronic lymphocytic thyroiditis. It is more common in women than men (ratio, five to one) and is most common in the fifth and sixth decades, when the prevalence of antithyroid antibodies in women is 10%. Classic Hashimoto’s disease, with an enlarged, firm, bosselated thyroid gland, is less common than the atrophic form. Both are characterised by the presence of serum antithyroid antibodies. Whereas levels of thyroglobulin and microsomal antibodies have conventionally both been measured, the sensitivity and specificity of thyroid peroxidase antibody assays make this the only test now required.
Around the world, iodine deficiency still remains the predominant cause of hypothyroidism. Mild iodine deficiency is re-emerging in Australia (possibly related to decreased consumption of iodised salt), but apparently not yet to a level to cause hypothyroidism.21 Public health measures to address this issue are likely to be needed, including monitoring of iodine intake in the population. More widespread use of iodised salt, both domestically and in manufactured food, may be sufficient to deal with the problem.
Congenital hypothyroidism has an incidence of about one in 4000 births, with thyroid agenesis and ectopia the main causes. Exposure of premature infants to iodine-containing antiseptics can result in transient hypothyroidism.22 Routine screening of all neonates by heel-prick blood sampling enables detection and treatment by the 10th day after birth. Appropriate thyroxine therapy, progressively adjusted by body weight, results in normal intellectual development.
Also known as mild thyroid failure and diminished thyroid reserve, “subclinical” hypothyroidism is defined biochemically by a raised serum TSH level in the presence of a serum free T4 level within the reference range. Because the T4 set point varies between individuals, it is possible for a serum free T4 level in the normal range for the population to be too low in a given individual. The risk of progression to frank hypothyroidism increases with increasing TSH levels (odds ratio for women, 8), significant titres of circulating autoantibody (odds ratio, 8) and even more with both (odds ratio, 38).2
In general, treatment is recommended if serum TSH level is more than 10 mU/L or progressively rising, or if thyroid antibodies or dyslipidaemia are present23 (see case report,Box 8).
Replacement thyroxine is the cornerstone of therapy for hypothyroidism. A dose of 1.6 μg per kg body weight daily is the average required in adults. The principal determinant of dose is lean body mass, so patients in old age may need as little as 50 μg/day. In patients with ischaemic heart disease, a low initial thyroxine dose is recommended (12.5–50 μg/day) to avoid exacerbating angina, but in some patients thyroxine replacement is impossible until coronary artery bypass surgery has been performed, with extreme attention to drug and fluid therapy.
As thyroxine has a half-life of 1 week, once-daily administration is fully adequate to maintain stable levels. It should be taken on an empty stomach, separately from other drugs.24 Dose should not be adjusted until after a minimum of three to five half-lives to allow a steady state to be attained. In primary hypothyroidism, normalisation of serum TSH level is the best biochemical marker of adequate therapy. Occasionally, symptoms of hypothyroidism appear to persist when TSH level is at the upper end of the reference range, and dose adjustment to achieve the mean normal TSH level of 1–2 mU/L1 is recommended. In pregnancy, the dose may need to be increased. Maintaining a normal TSH level is important for fetal development.25
Combined thyroxine and liothyronine (T3) therapy is being promoted, particularly on some Internet sites, for patients whose symptoms persist despite apparently adequate thyroxine therapy. There are few published data to support this practice,26 and a recent Australian study showed no benefit.27 Thus, it should be discouraged in the absence of evidence of benefit, with thyroxine alone remaining standard therapy.
In patients with hypothalamopituitary hypothyroidism, serum TSH level is not a valid biochemical indicator of adequate replacement, and it is usual to adjust the thyroxine dose to achieve an approximate mean normal serum level of free T4.
1: Interpretation matrix for thyroid function results*
|
High T4 |
Normal T4 |
Low T4 |
||||||||
High TSH |
In vivo or in vitro artefact Pituitary hyperthyroidism [TSHoma] Thyroid hormone resistance |
Mild thyroid failure (primary) (also termed subclinical hypothyroidism and diminished thyroid reserve) |
Primary hypothyroidism |
||||||||
Normal TSH |
As above Sampling within 6 h of thyroxine dose |
Normal (in patients taking thyroxine, TSH > 3 mU/L may indicate subtle underreplacement) |
Pituitary or hypothalamic hypothyroidism Severe non-thyroidal illness |
||||||||
Low TSH |
Hyperthyroidism (for this diagnosis, TSH must be suppressed rather than just low) |
Subclinical hyperthyroidism Subtle thyroxine overreplacement Thyroid autonomy (multinodular goitre or autonomous functioning thyroid nodule) Non-thyroidal illness |
Pituitary or hypothalamic hypothyroidism Severe non-thyroidal illness |
||||||||
T4 = serum free thyroxine. TSH = thyroid stimulating hormone. * The Health Insurance Commission restricts financial reimbursement to serum TSH alone, except in specified clinical circumstances, when additional measurements of free T4 and free triiodothyronine (T3) are sanctioned. We suggest that practitioners routinely request “thyroid function tests” and provide clear clinical information to enable the laboratory to perform additional tests if justified. The information should include the suspected condition (especially if hypopituitarism) and medications (eg, thyroxine, carbimazole or propylthiouracil, amiodarone or phenytoin). |
|||||||||||
2: Causes of hyperthyroidism
|
|
||||||||||
hCG = human chorionic gonadotropin. TSH = thyroid stimulating hormone. |
|||||||||||
4: Case report — hyperthyroidism
Presentation: A 25-year-old woman presented with a 6-month history of tremor, heat intolerance, irritability and weight loss of 4 kg. She had a small, diffuse, non-tender goitre (less than twice normal size), a pulse rate of 104 bpm, and slightly tremulous, warm, moist hands but no proximal myopathy. She had no personal or family history of thyroid disease. Fertility was not a current issue.
Investigations: Serum free thyroxine (T4) level was 34 pmol/L (reference range [RR], 9.3–23.8 pmol/L); free triiodothyronine (T3) level was 12 pmol/L (RR, 1.8–6.0 pmol/L); thyroid stimulating hormone (TSH) level was < 0.03 mU/L (RR, 0.4–4.7 mU/L); and thyroid peroxidase antibody level was 250 U/mL (RR, < 35 U/mL).
Comment: Radionuclide thyroid scanning will not assist management in the absence of a reasonable need to exclude subacute thyroiditis and in the presence of goitre.
Management: Graves disease was diagnosed, and carbimazole (10 mg twice daily) prescribed.
At 3-week review, the patient was feeling better, and free T4 level was 20 pmol/L; free T3, 6.5 pmol/L; and TSH, still < 0.03 mU/L. The carbimazole dose was reduced to 15 mg/day, and a further 3-week review scheduled.
Comment: There was a clinical and biochemical response, but free T3 level was still high. Dose reduction by 25%–50% was necessary to avoid overshoot to hypothyroidism.
At 6-week review, the patient was feeling much better, and her pulse rate was 76 bpm. Free T4 level was 16 pmol/L; free T3, 4.8 pmol/L; and TSH, 0.03 mU/L. The carbimazole dose was reduced to 10 mg/day as a single dose. The options for longer-term therapy were now discussed in detail with the patient.
Comment: Serum TSH can remain suppressed for weeks to months after euthyroidism is restored and therefore cannot be used to guide acute antithyroid drug therapy. Carbimazole is effective in a once-daily dose for maintenance, assisting compliance. When patients are euthyroid and well, the longer-term options for therapy should be discussed.
The patient elected to have a thionamide course. The dose was reduced and maintained at 5 mg/day in subsequent visits, with thyroid function monitoring every 2–3 months. After 18 months therapy was ceased.
Comment: Thyroid function should then be checked at decreasing frequencies indefinitely (eg, at 1 month, then after a further 2 months, 3 months, 6 months, and then annually) or if symptoms develop.
Three years later, the patient wished to get pregnant and enquired about possible thyroid problems. She was clinically euthyroid.
Comment: Thyroid function should be checked, not only to exclude subclinical hyperthyroidism, but also hypothyroidism, which is part of the natural history of Graves disease and can have a subtle adverse effect on fertility and fetal development. If the patient is euthyroid without prior ablative therapy, then assay of TSH-receptor antibodies is unnecessary, but may be useful in predicting the risk of neonatal hyperthyroidism. Thyroid function should be monitored, especially in the first trimester and particularly post partum, and the patient should be reminded about postpartum recurrence.
7: Causes of hypothyroidism
Autoimmune lymphocytic thyroiditis
Post-ablative therapy
Transient
Drug-induced
|
Iodine-associated
Infiltrative
Neonatal/congenital
Secondary
Other
|
||||||||||
TSH = thyroid stimulating hormone. |
|||||||||||
8: Case report — subclinical hypothyroidism
Presentation: A 52-year-old woman presented with tiredness, increasing over the previous 6 months. She was perimenopausal but did not have severe vasomotor symptoms. She was not depressed, and there were no significant physical findings.
Investigations: Routine laboratory tests excluded anaemia and iron deficiency, but serum free thyroxine (T4) level was 11.9 pmol/L (reference range [RR], 9.3–23.8 pmol/L); and thyroid stimulating hormone (TSH) level was 8.2 mU/L (RR, 0.3–4.7 mU/L). On review of specific symptoms, the patient felt her skin was a little dry and her mental concentration had deteriorated. She had chronic constipation.
Comment: This is a common problem. Symptoms are mild, non-specific and shared by many in the population who are not hypothyroid.
Repeat thyroid function tests confirmed earlier results, with free T4, 12.2 pmol/L; and TSH, 7.6 mU/L. Thyroid peroxidase antibodies were present at 1200 U/mL (RR, < 35 U/mL). Serum lipid tests showed cholesterol, 5.7 mmol/L (RR, < 5.5 mmol/L); high density lipoprotein, 1.2 mmol/L (RR, > 1.0 mmol/L); and triglycerides, 1.8 mmol/L (RR, < 2.0 mmol/L).
Comment: The patient has subclinical hypothyroidism (also known as mild thyroid failure or diminished thyroid reserve). In the presence of circulating antithyroid antibodies (thyroid peroxidase or thyroid microsomal antibodies), the risk of progression to frank hypothyroidism is 5% per year. Hypothyroidism may be contributing to the hyperlipidaemia and to accelerated coronary disease.
The patient was convinced that hypothyroidism was the cause of her symptoms. She had read on an Internet site about severe problems from hypothyroidism and that combined thyroxine and liothyronine (T4/ T3) therapy is best.
Comment: The worth of therapy in these circumstances is unresolved. In its favour are a possible reduction in symptoms, early prevention of progression to frank hypothyroidism, and possible prevention of accelerated vascular disease.
Against giving therapy is that symptoms very often do not change, and compliance is therefore poor, lipid profile generally does not improve when TSH is < 10 mU/L, there is no good evidence that vascular disease risk is reduced, and therapy increases the risk of subclinical hyperthyroidism and loss of bone mineral density in postmenopausal woman.
General recommendations for asymptomatic patients are:
Patients with TSH < 10 mU/L and no antithyroid antibodies should be monitored, with therapy started if TSH level increases above 10 mU/L.
Patients with TSH 5–10 mU/L and antithyroid antibodies present could be treated, depending on patient preference. The best therapy is thyroxine alone, adjusted for a TSH level of 1–2 mU/L. Patients who choose not to be treated should have annual monitoring.
The patient elected to have treatment (thyroxine, 100 μg/day). After 4 months, her thyroid function results were normal (free T4, 15.8 pmol/L; TSH, 1.9 mU/L), but she felt no different. She elected to discontinue treatment, but, as she was positive for thyroid peroxidase antibodies, her GP recommended annual thyroid function testing.
- 1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4 and thyroid antibodies in the United States population (1988-1994): National Health and Nutrition Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 486-488.
- 2. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995; 43: 55-68.
- 3. Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyrotropin (TSH) assays. Clin Chem 1996; 42: 140-145.
- 4. Stockigt JR. Free thyroid hormone measurement. A critical appraisal. Endocrinol Metab Clin North Am 2001; 30: 265-289.
- 5. Chen QY, Huang W, She JX, et al. HLA-DRB1*08, DRB1*03/DRB3*0101, and DRB3*0202 are susceptibility genes for Graves disease in North American Caucasians, whereas DRB1*07 is protective. J Clin Endocrinol Metab 1999; 84: 3182-3186.
- 6. Torring O, Tallstedt L, Wallin G, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine — a prospective, randomized study. J Clin Endocrinol Metab 1996; 81: 2986-2993.
- 7. Walsh JP. Management of Graves’ disease in Australia. Aust N Z J Med 2000; 30: 559-566.
- 8. Maugendre D, Gatel A, Campion L, et al. Antithyroid drugs and Graves’ disease – prospective randomized assessment of long-term treatment. Clin Endocrinol (Oxf) 1999; 50: 127-132.
- 9. McIver B, Rae P, Beckett G, et al. Lack of effect of thyroxine in patients with Graves’ hyperthyroidism who are treated with an antithyroid drug. N Engl J Med 1996; 334: 220-224.
- 10. Graham GD, Burman KD. Radioiodine treatment of Graves’ disease. An assessment of its potential risks. Ann Intern Med 1986; 105: 900-905.
- 11. Martin FI, Deam D. Hyperthyroidism in elderly hospitalised patients. Clinical features and treatment outcomes. Med J Aust 1996; 164: 200-203.
- 12. Ross DS, Ridgway EC, Daniels GH. Successful treatment of solitary thyroid nodules with relatively low-dose iodine-131, with low prevalence of hypothyroidism. Ann Intern Med 1984; 101: 488-490.
- 13. Fradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine (Baltimore) 1983; 62: 1-20.
- 14. Wong R, Cheung W, Stockigt JR, Topliss DJ. Heterogeneity of amiodarone-induced thyrotoxicosis: evaluation of colour-flow Doppler sonography in predicting therapeutic response. Intern Med J 2003; 33: 420-426.
- 15. Sawin CT, Geller A, Kaplan MM, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994; 331: 1249-1252.
- 16. Schneider DL, Barrett-Connor EL, Morton DJ. Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. JAMA 1994; 271: 1245-1249.
- 17. Cohen JH 3rd, Ingbar SH, Braverman LE. Thyrotoxicosis due to ingestion of excess thyroid hormone. Endocr Rev 1989; 10: 113-124.
- 18. Beck-Peccoz P, Brucker-Davis F, Persani L, et al. Thyrotropin-secreting tumors. Endocr Rev 1996; 17: 610-638.
- 19. Wynne AG, Gharib H, Scheitnauer BW, et al. Hyperthyroidism due to inappropriate secretion of thyrotropin in 10 patients. Am J Med 1992; 92: 15-20.
- 20. Hamblin PS, Dyer SA, Mohr VS, et al. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 1986; 62: 717-722.
- 21. Eastman CJ. Where has all our iodine gone? Med J Aust 1999; 171: 455-456.
- 22. Smerdely P, Boyages SC, Waite K, et al. Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birthweight infants. Lancet 1989; 2: 661-664.
- 23. Ayala AR, Danese MD, Ladenson PW. When to treat mild hypothyroidism. Endocrinol Metab Clin North Am 2000; 29: 399-415.
- 24. Chopra IJ, Baber K. Treatment of primary hypothyroidism during pregnancy: is there an increase in thyroxine dose requirement in pregnancy? Metabolism 2003; 52: 122-128.
- 25. Haddow JE, Palomakei GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341: 549-555.
- 26. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 1999; 340: 424-429.
- 27. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 2003; 88: 4543-4550.





Abstract
The most common cause of hyperthyroidism in Australia is Graves disease, caused by a defect in immunoregulation in genetically predisposed individuals, leading to production of thyroid-stimulating antibodies.
Each of the three modalities of therapy for Graves disease — thionamide drugs, subtotal or total thyroidectomy, and radioactive iodine ablation — can render the patient euthyroid, but all have potential adverse effects and may not eliminate recurrences.
Hypothyroidism occurs in about 5% of the adult population; most present with “subclinical” hypothyroidism (mild thyroid failure), characterised by raised levels of serum thyroid stimulating hormone (TSH) but normal free thyroxine (T4).
The most common cause of hypothyroidism in Australia is autoimmune chronic lymphocytic thyroiditis, characterised by raised circulating levels of thyroid peroxidase antibody.
Symptoms and signs of hypothyroidism are often mild or subtle and, when there is clinical suspicion, thyroid function tests are needed; if serum TSH level is raised, free T4 and thyroid peroxidase antibody should be measured.
Replacement therapy with thyroxine is the cornerstone of therapy (1.6 μg/kg lean body weight daily, taken on an empty stomach); combination therapy with thyroxine and liothyronine (T3) is promoted, but there is little evidence of its clinical benefit.
Despite the development of highly sensitive laboratory tests, clinical assessment and judgement remain paramount