Celecoxib, the first COX-2-selective non-steroidal anti-inflammatory drug (C2SN) to be developed, came onto the Australian market in 1999, and was listed on the Pharmaceutical Benefits Scheme (PBS) in August 2000. Rofecoxib, the second C2SN to come onto the market, was listed on the PBS in February 2001. The initial restrictions placed on the prescribing of these agents were, for celecoxib, “chronic arthropathies (including osteoarthritis) with an inflammatory component”, and for rofecoxib, “treatment of chronic osteoarthritis with an inflammatory component”.
Cyclooxygenase-1 (COX-1) inhibition in the gastrointestinal tract (GIT) was postulated as the reason for the increased risk of serious upper-GIT ulcers, perforation or bleeding associated with traditional non-steroidal anti-inflammatory drug (NSAID) use.1 C2SNs were thought to have a better gastrointestinal safety profile than traditional NSAIDs, because they selectively inhibit COX-2, an immediate early gene product which is rapidly induced in response to inflammatory stimuli.2 The manufacturers of C2SNs sponsored very large clinical trials in patients with osteoarthritis and rheumatoid arthritis to assess the risk of a serious GIT event with these drugs compared with traditional NSAIDs. The new drugs proved equally efficacious, and appeared to be associated with a lower relative risk of serious upper GIT events.3,4
Soon after celecoxib was placed on the PBS, many Australian general practitioners (GPs) began to prescribe it, leading to a large number of adverse drug reaction (ADR) reports and a blow out in government expenditure.5-7 The aim of our study was to assess trends in prescribing of the new agents by GPs, and the profiles of general practice patients who were prescribed COX-2-selective NSAIDs during the early adoption phase.
The General Practice Research Network (GPRN)8 comprises a national sample of Australian GPs who provide deidentified electronic longitudinal patient records. Overall prescription rates per 1000 encounters for traditional NSAIDs, celecoxib and rofecoxib, and meloxicam, were calculated using all of the data (from 437 GPs and 5 957 768 patient encounters) from all GPRN participants for the 3 years from 1 September 1999 to 30 September 2002.
Initially, two cohorts were identified for further analysis, comprising patients who had received their first prescription for either celecoxib or rofecoxib in the three months after their listing on the PBS. We also selected an additional cohort of patients who had received their first prescription for celecoxib after it had been listed on the PBS for six months to see if the pattern of use had changed.
To maximise the chances of obtaining comprehensive data for each patient, we included only the patients of GPs from practices where all members of the practice were also members of the research network. Thirty-eight practices where all GPs were enrolled with the GPRN were identified. The patients of ninety-six GPs from these practices were included in the cohorts for the C2SN analysis. The resulting database of 796 537 prescription records from January 1999 for the 96 GPs was searched for patients receiving their first prescription for celecoxib or rofecoxib.
To permit retrospective analysis, inclusion in the cohorts was restricted to patients whose records had prescription data available for the 12 months preceding their first C2SN prescription.
The first celecoxib cohort (Cohort 1) comprised 2366 patients who received their first prescription for celecoxib between 1 August and 31 October 2000. The second celecoxib cohort (Cohort 2) comprised 640 patients who received their first celecoxib prescription between 1 February and 30 April 2001. The rofecoxib cohort (Cohort 3) comprised 608 patients who received their first prescription for rofecoxib also between 1 February and 30 April 2001.
Data extracted from the electronic health records of patients in these three cohorts included patient age and sex, reason for C2SN prescription, pain medications prescribed in the previous 12 months, coprescription of aspirin, and coprescription of other medications which are known to adversely affect renal function when prescribed together with C2SNs (ie, diuretics, angiotensin-converting enzyme inhibitors [ACEI], angiotensin-2 receptor antagonists [AT2A]).9
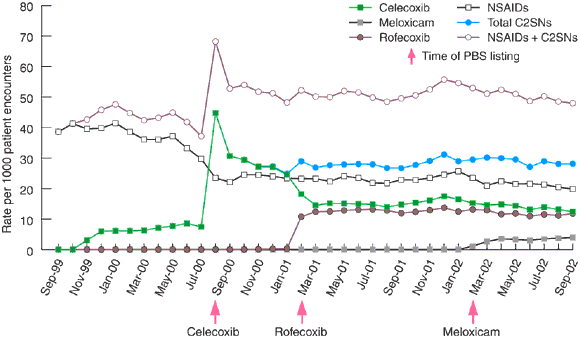
Prescriptions written for celecoxib increased dramatically from August 2000, when the drug was first made available on the PBS (Box 1). In the first month, celecoxib was the most frequently prescribed item among all GPRN doctors, accounting for 44.8 prescriptions written per 1000 patient encounters, and overtaking total prescriptions for all NSAIDs. This rate gradually fell over the next 7 months, stabilising at about 15 prescriptions per 1000 patient encounters. Prescriptions for rofecoxib increased, but not as dramatically, and the rate plateaued at about 13 prescriptions per 1000 patient encounters. Rate of prescribing of traditional NSAIDs did not fall after rofecoxib was listed on the PBS in February 2001, although the prescription rates for celecoxib decreased. The sum of the prescribing rates for C2SNs and traditional NSAIDs increased to a level about 20% higher than rates for traditional NSAIDs alone had been before C2SNs became available on the PBS.
Once enrolled in the GPRN, GPs are required to enter their reason the first time they prescribe a particular drug for a patient. However, as we used some retrospective prescribing data (from before GPs were enrolled), reasons were not always recorded. Thus, 38.9% of patients in Cohort 1 for whom C2SNs were prescribed had reasons for prescription recorded, increasing to 87.7% and 93.1% in Cohorts 2 and 3, respectively.
In all three cohorts, “osteoarthritis” or “arthritis” were the most frequently recorded reasons, and, together, accounted for between 37% and 47% of patients being prescribed C2SNs in each cohort (Box 2). Rheumatoid arthritis was recorded as the reason for prescribing C2SNs for less than 3% of patients in any cohort, reflecting the low prevalence of this condition in the population. Remaining reasons were primarily musculoskeletal conditions.
Between 36.7% and 61.3% of patients in the three cohorts had not received a prescription for any pain medication in the year before being prescribed a C2SN (Box 3). Varying proportions of patients in the three cohorts received prescriptions for traditional NSAIDs, paracetamol, combinations of paracetamol and codeine, or other pain medications, in the 12 months preceding their first C2SN prescription. Fewer than 33.2% of patients in any cohort had been prescribed a traditional NSAID, and fewer than 47.7% had been prescribed paracetamol or a paracetamol and codeine combination; 31.6% of patients in the rofecoxib cohort had previously been prescribed celecoxib.
Two years after the introduction and rapid adoption of celecoxib and rofecoxib, there has been an overall growth in the market for anti-inflammatory medications. In our general practice study, prescribing rates per 1000 consultations for C2SNs plus traditional NSAIDs increased by about 20%.
There are several caveats in interpreting data from the GPRN database. Firstly, it comprises only a sample of GPs (although GPRN participants are representative of Australian GPs overall8). Secondly, it is possible that datasets may be incomplete, as patients may visit more than one GP. A final caution relates to uncertainty of reasons for prescription recorded in the absence of recognised standard disease coding criteria. Notwithstanding these limitations, the GPRN allows overall prescribing rates of C2SNs and NSAIDs to be calculated; this cannot be done by using the Health Insurance Commission database, as it does not capture prescriptions for traditional NSAIDs that fall below the patient copayment.
The increase in COX-2 prescribing coincided with a period of energetic marketing to the medical profession, which promoted the message that the new C2SNs were “safer” than traditional NSAIDs.11 While the studies available at that time suggested that treatment with C2SNs was associated with a lower risk of a serious upper GI event, the adverse event profile was otherwise not appreciably different to that for traditional NSAIDs.3,4 In Australia and the United States, there was also an extensive campaign to promote celecoxib to consumers through television and newspaper coverage.12,13
Less than a third of the patients in any of our cohorts who were introduced to a C2SN had been prescribed a traditional NSAID in the previous year, and between 36.7% and 63.3% had not been prescribed any pain medication in the previous year. A large proportion of patients were aged under 65 years. Major recorded reasons for prescribing were osteoarthritis or arthritis, with few being for rheumatoid arthritis. Many patients appeared to have received C2SNs as first-line therapy. International and Australian guidelines published after the time of our study advise that C2SNs are most useful for patients who need maximum doses for a prolonged time, or for those aged over 65 years.9,14,15
A proportion of patients may have previously experienced gastrointestinal toxicity with traditional NSAIDs, but this could not be determined from our study. Nevertheless, the extent of C2SN prescribing in the absence of previous prescribed pain medication suggests that GPs were ready to adopt a newer “safer” agent for their patients whatever their actual level of GIT risk. Importantly, continued controversy over the methods and analyses of the CLASS3 and VIGOR4 trials, together with the current level of uncertainty over the absolute ratio of risk to benefit of C2SN therapy, indicate that assumptions of superior safety were premature.16-18
Another possible explanation for the scale of early use of these drugs may have been the way the restriction was worded in the PBS. It is debatable how strongly the existence of a restriction weighs with prescribers, as, unlike an authority wording, no special effort is required to obtain the drug for the patient. In any event, the initial restriction in the PBS listing for celecoxib (“chronic arthropathies [including osteoarthritis] with an inflammatory component”) did not differ from that for traditional NSAIDs. Whatever was intended, it seems no special message was received by prescribers through these listings (see Box 1). The fall in prescribing for celecoxib and the plateau in prescribing for both agents presumably reflected the inevitable reassessment of its effectiveness and safety after the initial marketing and promotion.
It is particularly interesting that a third of the patients in Cohort 3 had previously been prescribed celecoxib, suggesting that these patients did not tolerate or were not happy with the efficacy of this medication.
Our study also indicates the potential for adverse effects with widespread use of C2SNs. Terminology and acronyms used for the new agents have had many variants in the short time since they were released, reflecting the debate over the extent to which they are indeed a new drug class.19 However, their promotion as a new class may have left general practitioners without the reminder that the new agents, like the old, can cause renal impairment and fluid retention.20 The risk of these adverse effects is increased by concomitant therapy with diuretics, ACE inhibitors or AT2As, and is further exacerbated when ACE inhibitors or AT2As are taken together with diuretics and C2SNs or NSAIDs.7,20 Despite this, between 4.7% and 7.9% of patients in our three cohorts were being treated with this combination of three agents. These findings are consistent with those of a clinical audit undertaken with Australian rural GPs.21
More generally, the potential for adverse effects is greater when prescribing a new drug soon after its release onto the market. It has been shown that early adopters put their patients at risk of adverse effects.22 A recent study found that 10% of new drugs approved by the US Food and Drug Administration between 1975 and 1999 were either withdrawn from the market or acquired a “black box” warning (a US labelling device aimed at increasing drug safety) as a result of newly discovered adverse drug reactions.23 Indeed many adverse events, particularly those that are idiosyncratic and not expected from a drug’s pharmacological properties, may not manifest until a medication has been in routine clinical use for a number of years.
A number of lessons can be gleaned from the Australian experience of large-scale early adoption of celecoxib and rofecoxib by GPs. Intense drug promotion can create perceptions about medicines that strongly influence patterns of prescribing and use, yet may not be in line with best available evidence. Further, such rapid uptake can place patients at risk of adverse drug reactions and serious drug interactions through coprescribing. The Quality Use of Medicines (QUM) arm of the Australian National Medicines Policy dictates that prescribing should be judicious, safe, appropriate, and cost-effective.24 Our view is that transparency and collaboration between regulatory agencies, the pharmaceutical industry and organisations such as the National Prescribing Service are needed if new medications are to be prescribed and used in a way that enhances the health of individuals and also adheres to QUM principles.
1: Prescription rate per 1000 patient encounters of C2SNs and NSAIDs, showing the time of Pharmaceutical Benefits Scheme (PBS) listing for celecoxib, rofecoxib and meloxicam
2: Top 10 reasons for prescribing celecoxib and rofecoxib in study cohorts
|
Cohort 1* (2366 patients) |
|
Cohort 2† (640 patients) |
|
Cohort 3‡ (608 patients) |
||||||
Reason for prescribing |
n |
% (95% CI)§ |
|
n |
% (95% CI) |
|
n |
% (95% CI) |
|
||
Osteoarthritis |
253 |
27.5 (21.4–33.6) |
|
107 |
19.1 (13.2–24.9) |
|
158 |
27.9 (19.0–36.8) |
|
||
Arthritis |
183 |
19.9 (14.6–25.1) |
|
101 |
18.0 (12.8–23.2) |
|
103 |
18.2 (8.6–27.8) |
|
||
Pain other than knee/back |
105 |
11.4 (7.7–15.1) |
|
82 |
14.6 (10.2–19.0) |
|
78 |
13.8 (8.1–19.5) |
|
||
Backpain |
74 |
8.0 (5.2–10.9) |
|
61 |
10.9 (7.1–14.6) |
|
37 |
6.5 (3.9–9.2) |
|
||
Knee pain |
17 |
1.8 (0.6–3.1) |
|
24 |
4.3 (2.4–6.2) |
|
14 |
2.5 (0.7–4.2) |
|
||
Injury/sprain/strain |
18 |
2.0 (1.1–2.9) |
|
19 |
3.4 (0.5–6.3) |
|
10 |
1.8 (0.6–2.9) |
|
||
Sciatica |
16 |
1.7 (0.5–2.9) |
|
14 |
2.5 (1.2–3.8) |
|
9 |
1.6 (0.4–2.8) |
|
||
Arthralgia |
10 |
1.1 (0.3–1.9) |
|
10 |
1.8 (0.5–3.1) |
|
6 |
1.1 (0.0–2.3) |
|
||
Gout |
10 |
1.1 (0.5–1.7) |
|
9 |
1.6 (0.0–3.2) |
|
3 |
0.5 (0.0–1.1) |
|
||
Spondylosis |
30 |
3.3 (1.7–4.9) |
|
8 |
1.4 (0.0–2.8) |
|
21 |
3.7 (1.1–6.3) |
|
||
Rheumatoid arthritis |
10 |
1.1 (0.3–1.9) |
|
6 |
1.1 (0.0–2.1) |
|
15 |
2.7 (1.4–3.9) |
|
||
Tendonitis |
4 |
0.4 (0–0.9) |
|
6 |
1.1 (0.1–2.0) |
|
9 |
1.6 (0.2–3.0) |
|
||
Reason for script recorded |
921 |
38.9 |
|
561 |
87.7 |
|
566 |
93.1 |
|
||
* Celecoxib first prescribed Aug–Oct 2000. † Celecoxib first prescribed Feb–Apr 2001. ‡ Rofecoxib first prescribed Feb–Apr 2001. § Percentages are calculated as a proportion of prescriptions where a reason for prescribing was recorded by the general practitioner. |
|
||||||||||
3: Prescription for pain medications in the 12 months preceding first prescription for celecoxib or rofecoxib
|
Cohort 1* (2366 patients) |
|
Cohort 2† (640 patients) |
|
Cohort 3‡ (608 patients) |
||||||
Prescribed pain medication§ |
n |
% (95% CI) |
|
n |
% (95% CI) |
|
n |
% (95% CI) |
|||
Traditional NSAID¶ |
785 |
33.2 (29.4–36.9) |
|
124 |
19.4 (15.0–23.7) |
|
169 |
27.8 (22.4–33.2) |
|||
Celecoxib¶ |
— |
— |
|
— |
— |
|
192 |
31.6 (27.1–36.0) |
|||
Paracetamol¶ |
493 |
20.8 (17.5–24.2) |
|
98 |
15.3 (12.7–17.9) |
|
164 |
27.0 (20.0–34.0) |
|||
Paracetamol/codeine¶ |
419 |
17.7 (15.7–19.8) |
|
81 |
12.7 (8.8–16.5) |
|
126 |
20.7 (15.2–26.2) |
|||
Other pain medication¶,** |
182 |
7.7 (5.9–9.4) |
|
39 |
6.1 (4.4–7.8) |
|
89 |
14.6 (10.7–18.5) |
|||
No prescribed pain medication |
1095 |
46.3 (41.8–50.8) |
|
392 |
61.3 (55.4–67.1) |
|
223 |
36.7 (31.8–41.6) |
|||
* Celecoxib first prescribed Aug–Oct 2000. † Celecoxib first prescribed Feb–Apr 2001. ‡ Rofecoxib first prescribed Feb–Apr 2001. § Refers to the 12 months preceding the first prescription for celecoxib or rofecoxib. ¶ These groups are not mutually exclusive. ** Includes codeine alone, tramadol, combination dextropropoxyphene/paracetamol, and morphine. |
|||||||||||
4: Medications concomitantly prescribed with COX-2-selective non-steroidal anti-inflammatory drugs that potentially increase the risk of adverse renal events or bleeding
|
Cohort 1* (2366 patients) |
|
Cohort 2† (640 patients) |
|
Cohort 3‡ (608 patients) |
||||||
Coprescribed medication |
n |
% (95% CI) |
|
n |
% (95% CI) |
|
n |
% (95% CI) |
|||
Diuretic |
279 |
11.8 (10.2–13.4) |
|
52 |
8.1 (5.3–11.0) |
|
66 |
10.9 (8.2–13.5) |
|||
ACEIs |
273 |
11.5 (9.1–14.0) |
|
53 |
8.3 (6.4–10.1) |
|
60 |
9.9 (7.5–12.2) |
|||
AT2As |
132 |
5.6 (3.0–8.1) |
|
22 |
3.4 (1.6–5.3) |
|
45 |
7.4 (5.4–9.4) |
|||
Combination ACEI/diuretic |
97 |
4.1 (3.0–5.2) |
|
16 |
2.5 (1.5–3.5) |
|
27 |
4.4 (2.9–6.0) |
|||
Combination AT2A/diuretic |
54 |
2.3 (1.0–3.6) |
|
14 |
2.2 (1.1–3.3) |
|
21 |
3.5 (2.3–4.6) |
|||
Warfarin |
16 |
0.7 (0.3–1.1) |
|
6 |
0.9 (0.2–1.7) |
|
2 |
0.3 (0.0–0.7) |
|||
Aspirin |
183 |
7.7 (5.2–10.3) |
|
20 |
3.1 (1.7–4.5) |
|
47 |
7.7 (5.1–10.4) |
|||
ACEI = angiotensin-converting enzyme inhibitor; AT2A = angiotensin-2 receptor antagonist. * Celecoxib first prescribed Aug–Oct 2000. † Celecoxib first prescribed Feb–Apr 2001. ‡ Rofecoxib first prescribed Feb–Apr 2001. |
|||||||||||
Received 15 January 2003, accepted 18 June 2003
- Stephen J Kerr1
- Andrea Mant2
- Fiona E Horn3
- Kevin McGeechan4
- Geoffrey P Sayer5
- 1 National Prescribing Service, Sydney, NSW.
- 2 South East Health, Darlinghurst, NSW.
- 3 Health Communication Network, St Leonards, NSW.
In 1997, Associate Professor Andrea Mant provided consultancy advice on Quality Use of Medicines to Merck Sharp & Dohme.
- 1. Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996; 312: 1563-1566.
- 2. Hawkey CJ. COX-2 inhibitors. Lancet 1999; 353: 307-314.
- 3. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study. A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000; 284: 1247-1255.
- 4. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000; 343: 1520-1528.
- 5. Davies JA. Arthritis drug under siege. The Age (Melbourne) 6 Feb 2001; Sect: News: 1.
- 6. Your reports at work — drug safety in Australia. Aust Adverse Drug React Bull 2001; 20(4): 15-16.
- 7. Boyd IW, Mathew TH, Thomas MC. COX-2 inhibitors and renal failure: the triple whammy revisited. Med J Aust 2000; 173: 274.
- 8. Sayer GP, McGeechan K, Kemp A, et al. The General Practice Research Network: the capabilities of an electronic patient management system for longitudinal patient data. Pharmacoepidemiol Drug Safety 2003; 12: 483-489
- 9. Therapeutic guidelines: analgesic. 4th ed. Melbourne: Therapeutic Guidelines Limited; 2002.
- 10. SAS. Version 8 [computer program]. Cary, NC: SAS Institute Inc, 2002.
- 11. Mansfield P. Healthy scepticism about Pfizer and Searle’s promotion of celecoxib in Australia. Healthy Skepticism Int Ed March/April 2000; 18 (3/4). Available at: www.healthyskepticism.org/editions/IN0004.htm (accessed Dec 2002).
- 12. Davies J-A, Foley B. A drug that was sold on hope. The Age (Melbourne) 10 Feb 2001; Sect: News: 1.
- 13. Anderson WB. The media battle between Celebrex and Vioxx: influencing media coverage but not content. Public Relat Rev 2001; 27: 449-460.
- 14. Maetzel A, Krahn MD, Naglie G. Economic assessment: celecoxib and rofecoxib for patients with osteoarthritis and rheumatoid arthritis. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2002. Report No.: Technology Overview Number 6. Available at: www.ccohta.ca/ (accessed May 2003).
- 15. Guidance on the use of cyclo-oxygenase (COX) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis. London: National Institute for Clinical Excellence; July 2001. Report No.: NICE Technology Appraisal Guidance Number 27. Available at: www.nice.org.uk/pdf/coxiifullguidance.pdf (accessed May 2003).
- 16. Jones R. Efficacy and safety of COX 2 inhibitors. BMJ 2002; 325: 607-608.
- 17. Juni P, Rutjes AW, Dieppe PA. Are selective COX 2 inhibitors superior to traditional non steroidal anti-inflammatory drugs? BMJ 2002; 324: 1287-1288.
- 18. Lassere M, Johnson K. The power of the protocol. Lancet 2002; 360: 1620-1622.
- 19. Lipsky LP, Abramson SB, Crofford L, et al. The classification of cyclooxygenase inhibitors. J Rheumatol 1998; 25: 2298-2303.
- 20. The Australian COX-2-Specific Inhibitor (CSI) Prescribing Group. Considerations for the safe prescribing and use of COX-2-specific inhibitors [position statement]. Med J Aust 2002; 176: 328-331. <MJA full text>
- 21. Cutts C, LaCaze A, Tett S. A clinical audit of the prescribing of celecoxib and rofecoxib in Australian rural general practice. Br J Clin Pharmacol 2002; 54: 522-527.
- 22. Inman W, Pearce G. Prescriber profile and post-marketing surveillance. Lancet 1993; 342: 658-661.
- 23. Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287: 2215-2220.
- 24. National medicines policy 2000. Canberra: Commonweatlh Department of Health and Aged Care; 1999.





Abstract
Objective: To assess trends in the first two years of prescribing of COX-2-selective non-steroidal anti-inflammatory drugs (C2SNs) by Australian general practitioners.
Design: Retrospective analysis of deidentified electronic patient records from GPs enrolled in the General Practice Research Network (GPRN).
Setting and participants: Overall prescription rates for C2SNs and NSAIDs were assessed for all GPRN participants (437 GPs) between 1 September 1999 and 30 September 2002. Also, three cohorts of patients, with at least 12 months of prescription data, who received their first prescription for celecoxib between August and October 2000 (Cohort 1, 2366 patients), celecoxib between February and April 2001 (Cohort 2, 640 patients), and rofecoxib between February and April 2001 (Cohort 3, 608 patients) were selected for further analysis.
Main outcome measures: Age and sex of patients; reason for prescription; previously prescribed pain medications and concomitant use of medications that could predispose to an adverse renal or bleeding event.
Results: Prescriptions for C2SNs increased dramatically after they were listed on the Pharmaceutical Benefits Scheme (PBS). C2SN prescriptions for patients aged less than 65 years accounted for 52.6%, 59.5% and 50.7% of those in Cohorts 1, 2 and 3, respectively; large numbers of patients in the study cohort had reasons recorded for prescription that did not comply with PBS restrictions, and between 36.7% and 61.3% of patients in the three cohorts had not received a prescription for any pain medication in the year before being prescribed a C2SN. Between 4.7% and 7.9% were coprescribed drugs that could cause renal complications.
Conclusions: Rapid, early adoption of C2SNs by Australian GPs has resulted in prescribing and drug use patterns that were not in accord with quality use of medicine (QUM) principles.