In the past decade, two major advances have improved the care of patients with type 1 diabetes. First, we understand the unequivocal relationship between metabolic control and vascular complications;1-4 suboptimal blood glucose control has a lasting harmful effect even if control improves later. Second, the development of new forms of insulin with more rapid onset or longer duration of action (insulin analogues) and new forms of delivery (eg, continuous pumps and aerosol sprays), as well as advances in glucose monitoring, provide more options for those affected.
Because of the relationship between suboptimal glycaemic control and vascular complications, intensive management to minimise hyperglycaemia is now recommended for all patients. This may include more frequent administration of insulin (up to four times daily) or use of different insulin types (eg, rapid- and long-acting insulin analogues), as well as more frequent blood glucose measurements and changes to insulin dose than in conventional therapy.
Intensive schedules need to be individualised to suit patient age and lifestyle. For example, adults and adolescents generally need intermediate-acting insulin before bed for night-time control. Some school-aged children need short-acting insulin at afternoon tea rather than lunch-time to prevent late-afternoon hyperglycaemia, while many preschool-aged children can be managed with one dose of intermediate-acting insulin in the morning and small doses of rapid-acting insulin analogues (see below) to prevent hyperglycaemia later in the day. The imminent availability of long-acting insulin analogues will change these schedules considerably. Insulin pumps may be useful for some patients with frequent hypoglycaemia or hypoglycaemic unawareness.
Intensive therapy is more demanding for patients and their families, and adherence is obviously critical to its success. Patient views are therefore central to management decisions. For example, an adolescent who struggles to comply with two injections a day is unlikely to manage four injections (see case report, Box 1). However, for most patients, the demands of achieving good control are less than the consequences of poor control.5 In adolescents, good control improves quality of life and reduces the burden perceived by their parents.5
The limiting factor preventing ideal glycaemic control remains hypoglycaemia. Glucagon secretion is blunted early in the course of type 1 diabetes, increasing the patient’s vulnerability to hypoglycaemia. Furthermore, the blood glucose threshold for release of catecholamines, which both stimulate glucose production and produce warning symptoms such as sweating and trembling in response to hypoglycaemia, is lowered in patients with better glycaemic control, even more so during sleep.6 Continuous glucose monitoring devices reveal the frequency of nocturnal hypoglycaemia and the limitations of conventional blood glucose monitoring. Both the new insulin analogues and continuous subcutaneous insulin therapy hold promise of improving control without the attendant risk of hypoglycaemia.
In the past, the pharmacokinetic characteristics of insulin preparations have been modified by mixing with substances that delay absorption (eg, protamine and zinc) and by varying crystal size. Recombinant DNA technology has now made possible the creation of analogues of human insulin with altered pharmacokinetic characteristics (Box 2).
Rapid-acting analogues: These are also known as rapid-onset and ultra-short-acting insulins and include insulin aspart and insulin lispro. They were created by modifying the amino acid sequence of the insulin B chain (substituting aspartic acid for proline at position 28 [aspart] and reversing proline and lysine at positions 28 and 29 [lispro]).
These analogues have the advantages that:
they follow better the rise in blood glucose level after eating than conventional short-acting insulins, thereby reducing postprandial hyperglycaemia and between-meals hypoglycaemia;
their very rapid onset of action allows them to be injected immediately before meals or even after eating, which is especially useful in young children with erratic eating patterns.
Their disadvantages are:
shorter duration of action than traditional short-acting human insulins, which could cause preprandial hyperglycaemia.
The short-acting analogues are available on the Pharmaceutical Benefits Scheme.
Long-acting analogues: These were created by substituting and adding amino acids to the insulin molecule (glargine) and by adding a fatty acid chain, which enhances binding to albumin (detemir).
The long-acting analogues have the advantages of:
more reproducible absorption than conventional long-acting insulins;
a flat dose profile with a low peak of action, which provides more predictable background control than the intermediate-acting insulins, without the unwanted peaks of action around lunchtime and during the night.
Initial studies indicate that the long-acting analogues reduce the risk of nocturnal hypoglycaemia and produce a modest reduction in fasting blood glucose levels compared with intermediate-acting preparations.7 Neither is as yet available on the Pharmaceutical Benefits Scheme (PBS). There are few data on the relative benefits of administration by pump versus multiple daily injections for glargine or detemir as the basal insulin, but better night-time blood glucose control with pump therapy has recently been described.8
Pump therapy: The effectiveness of continuous subcutaneous insulin infusion (pump therapy) has been rediscovered (Box 3). First used in the 1970s and 1980s, pump therapy has been reintroduced with improved technology. Small amounts of a rapid-acting insulin analogue are infused, usually into the abdomen or buttocks, at a basal rate, which can be varied, with extra boluses calculated for each meal and snack. The major advantages are less variable absorption and improved insulin pharmacokinetics. Varying the basal rate can help regulate overnight blood glucose levels. Pump therapy is suitable for motivated patients, particularly those with frequent hypoglycaemic episodes or hypoglycaemic unawareness, as most studies show reduced hypoglycaemia.9,10 Because the infused insulin is rapid-acting, mechanical interruption of the pump can rapidly lead to ketosis, which is of particular concern in pregnancy. Pump therapy must therefore be accompanied by frequent blood glucose monitoring. Private health insurance companies now subsidise the cost of the pump and some consumables, but there is no government funding. As for all therapies, it is essential that the patient is central to the choice of pump therapy. This is also important when the patient is a child or adolescent, whose views may differ from those of their parents.
Aerosols: Aerosolised insulins for delivery by inhalation are under active investigation, with Phase III studies completed. These insulins provide effective cover for meals in combination with once-daily, long-acting, subcutaneous insulin. However, aerosol delivery requires six times as much insulin for the same effective dose as subcutaneous injection, which may create a cost barrier to widespread use. Also, the long-term safety of delivering large amounts of insulin to the alveolae is not known.
At present, all systems deliver insulin to the systemic circulation rather than the enteroportal circulation. Systemic delivery contributes to the insulin resistance associated with obesity and adolescence and is a major barrier to physiological insulin replacement. However, a method of accessing the enteroportal circulation is not currently in sight.
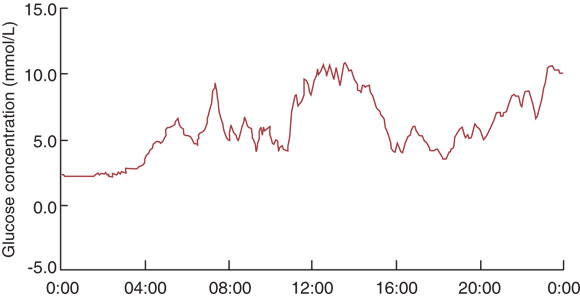
Continuous blood glucose monitoring: Intermittent measurements of capillary blood glucose give a limited glimpse of blood glucose levels, which may fluctuate widely over 24 hours. The recent introduction of systems for continuous blood glucose monitoring is an exciting advance11,12 (Box 4). Continuous monitoring reveals postprandial fluctuations in glucose level and asymptomatic nocturnal hypoglycaemia, and is likely to be especially useful in programming overnight basal insulin rates for pump therapy. Continuous monitoring systems available in Australia measure interstitial blood glucose via an indwelling cannula in the abdomen or buttocks. However, they are expensive ($5000 or more) and, at present, are most applicable for use by diabetes centres which lend devices to patients for restabilisation. Devices under investigation have alarms to alert patients to glucose levels outside a target range and provide immediate read-outs.
Non-invasive blood glucose monitoring: Frequent automatic glucose readings can be obtained non-invasively through the process of reverse iontophoresis, in which a low electric current pulls glucose molecules through the skin for collection in a gel disc. A device that is worn like a wristwatch is approved for patients aged over 7 years in the United States, but its cost (about US$1000 for the device and more than US$100 per disposable 12-hour sensor) limits more widespread use. It is not yet available in routine practice in Australia.
HbA1c measurement: Measurement of glycosylated haemoglobin (HbA1c) remains the criterion standard to judge the outcome of diabetes management. Rapid measurement with a desktop device expedites assessment and education. Results of longitudinal studies now allow accurate prediction of the risk of vascular complications for a given HbA1c level3,4 (Box 5). This can be very useful when counselling patients. For example, a teenager can be advised that large trials show that reducing the HbA1c level from 9% to 8% and maintaining the reduction during adolescence reduces the risk of proliferative and severe non-proliferative retino-pathy in young adulthood by 70%–80%.3
Education and psychological support: With the advent of intensive therapy and its greater demands on patients and their families, counselling and education are becoming even more important in achieving compliance, particularly in adolescents. However, as long-term risk of vascular complications can be predicted more accurately from HbA1c level, it is easier for doctors to counsel and encourage intensive management.1-4 Furthermore, as doctors have become more certain about the need for intensive management, it is our impression that patients and their families have accepted and coped better with it. Indeed, mean HbA1c levels in Australian children have improved from 10% in the 1980s to around 8% in 2003.14
Coping-skills training has been shown to improve metabolic control and quality of life in adolescents.15 This intervention, like all successful interventions in type 1 diabetes, needs to be intensive, and support must be sustained.
The indispensable role of the diabetes educator and dietitian in the multidisciplinary team has long been recognised. Dietary management and education is now more comprehensive and includes the concept of the glycaemic indices of food.16 Educators’ roles are becoming more specialised, both for different age groups and, as technology progresses, for different monitoring and delivery systems.
New use of old drugs: Adding metformin to the treatment regimen for type 1 diabetes can improve metabolic control by reducing insulin resistance, a problem in both obese patients and adolescents with diabetes, in whom the physiological insulin resistance of puberty is exaggerated, necessitating high insulin doses.17 However, metformin is suitable only for patients with low alcohol intake and normal renal and hepatic function who remain compliant with insulin therapy.
Glucagon in small frequent doses (< 0.15 mg up to 6-hourly) can prevent hypoglycaemia (and resulting hospital admissions) in patients with reduced oral intake, including children with viral gut infections.18
Type 2 diabetes mellitus generally forms part of the “metabolic syndrome”, which is characterised by insulin resistance, obesity and a range of cardiovascular risk factors, as discussed previously in this series.19 Advances in management of type 2 diabetes include recognition of the need for early and aggressive management of insulin resistance and associated abnormalities, such as dyslipidaemia, hypertension and albuminuria.
Therapy for type 2 diabetes should be tailored to the pathological process that is most prominent at different stages of the disease (see case report, Box 6).
Increase insulin sensitivity: Early in the disease process, the aim should be to target insulin resistance with diet and exercise plus metformin therapy. A weight loss of 5 kg leads to a 25%–50% reduction in insulin resistance (J Prins, unpublished data). Metformin is tolerated by most patients if started at a low dose (eg, 250–500 mg/day), which is slowly increased depending on blood sugar response and tolerance.
The thiazolidinediones (rosiglitazone and pioglitazone) are newer insulin-sensitising agents which are effective, but have the disadvantage of causing some weight gain and fluid retention. However, unlike metformin, they are safe in patients with renal impairment. They are widely used overseas but are not currently available on the PBS.20
Increase circulating insulin: If strategies to increase insulin sensitivity are ineffective, the next step is to increase circulating insulin level. Oral agents currently available in Australia for this purpose are the sulfonylureas and the newer glitinides. Metformin should be continued if the patient is obese.
As sulfonylureas are potent and long-acting, hypoglycaemia is a significant clinical problem, but can be avoided with careful dose titration. Sulfonylureas cause weight gain of a few kilograms in most patients and lose efficacy with time, either because of increasing insulin resistance (usually due to the weight gain) or “secondary” pancreatic β-cell failure. Other than half-life differences, there is little to distinguish later-generation sulfonylureas.
Glitinides (eg, repaglinide) are short-acting drugs that increase insulin secretion. They are well tolerated but less potent in clinical practice than the sulfonylureas. Their advantage lies in their short half-life, making hypoglycaemia less common, but pre-meal dosing is a disadvantage for many patients. Glitinides are not currently available on the PBS.
Exogenous insulin: If diabetic control remains, or becomes, suboptimal when taking oral combination therapy with metformin and a sulfonylurea, then insulin is indicated.21,22 As evidence supporting the benefits of good diabetic control is now overwhelming,13 any delay in introducing insulin in these patients is now considered unacceptable. It is important to educate patients that insulin is usually necessary because of natural progression of the disease and not necessarily because of any “failure” on their part. It is therefore advisable to introduce the concept well before necessary. A good “selling point” is that insulin often substantially improves how patients feel.
With currently available insulin preparations and administration devices, it is straightforward to develop an acceptable “physiological” insulin-replacement regimen for almost all patients.21,22 Newer analogue insulins (including mixtures) have a definite place in the management of type 2 diabetes and contribute to the excellent choices available.
There is little point in continuing sulfonylurea or glitinide therapy after introducing insulin. Advantages of ceasing oral agents are simplification of therapy, less chance of drug interaction, reduced costs and improved compliance. However, metformin should be continued in obese patients, as it helps maintain weight and allows lower insulin doses to be used. Pioglitazone is the only insulin-sensitiser available for combination with insulin in Australia for patients with renal impairment.
Insulin analogues: Many of the new insulin analogues will have a place in management of type 2 as well as type 1 diabetes.
Dual PPARγ/α agonists: The nuclear hormone receptor PPARγ (peroxisome proliferators-activated receptor) is involved in glucose and, to a lesser extent, lipid metabolism and is a target of the thiazolidinediones (glitizones). Drugs are currently under trial which act both on this receptor and a related receptor involved in lipid metabolism, which is targeted by the fibrates. These dual PPARγ/a agonists will probably have an important role in managing type 2 diabetes and the metabolic syndrome within a few years.
Orlistat: This lipase inhibitor is currently indicated for use in obesity. Clinical trials have demonstrated its usefulness as an insulin-sensitising agent, with the added benefit of weight management.23 Overall efficacy appears similar to that of metformin or sulfonylurea.
Insulin secretagogues: These include the gut-derived hormones (incretins), such as glucagon-like peptide-1 (GLP1) and gastric inhibitory peptide. GLP-1 analogues and receptor agonists are currently under clinical trial and show definite promise for patients with type 2 diabetes. Administration is parenteral, but initial trial results demonstrate efficacy in blood sugar control, minimal risk of hypoglycaemia, and the associated benefit of weight loss. A related approach is oral administration of dipeptidase IV inhibitors, which effectively increase GLP-1 levels.24
2: New and commonly used insulin preparations in Australia*
Preparation |
Insulin type |
Onset of action |
Maximum effect (h) |
Duration of action (h) |
|||||||
New insulin analogues† |
|
|
|
||||||||
Rapid-acting analogues |
|
|
|
||||||||
Humalog |
Lispro |
15 min |
1–2 |
3.5–4.5 |
|||||||
NovoRapid |
Aspart |
10–20 min |
1–3 |
3–5 |
|||||||
Biphasic analogue mixtures |
|
|
|
||||||||
Humalog Mix25 |
Lispro + lispro protamine suspension |
15 min |
2 |
24 |
|||||||
NovoMix 30 |
Aspart + aspart protamine crystallised |
10–20 min |
1–4 |
24 |
|||||||
Long-acting analogues‡ |
|
|
|
||||||||
Lantus |
Glargine |
1–2 h |
6 +§ |
24 + |
|||||||
Not yet named |
Detemir |
1–2 h |
6–8 |
20 |
|||||||
Conventional human insulins |
|
|
|
||||||||
Short-acting insulins |
|
|
|
||||||||
Actrapid |
Neutral |
30 min |
2.5–5 |
8 |
|||||||
Humulin R |
30 min |
2–4 |
6–8 |
||||||||
Intermediate-acting insulins |
|
|
|
||||||||
Humulin NPH |
Protamine suspension |
1h |
4–10 |
16–18 |
|||||||
Protaphane |
1.5 h |
4–12 |
24 |
||||||||
Humulin L |
Zinc suspension (lente) |
2 h |
6–12 |
24 |
|||||||
Monotard |
2.5 h |
7–15 |
22 |
||||||||
Long-acting insulins |
|
|
|
||||||||
Humulin UL |
Microcrystallinezinc suspension (ultralente) |
2 h |
6–20 |
24 + |
|||||||
Ultratard |
4 h |
8–24 |
28 |
||||||||
Biphasic mixtures |
|
|
|
||||||||
Humulin 30/70 |
Mixture of a neutral insulin and a protamine suspension |
30 min |
2–12 |
16–18 |
|||||||
Mixtard 30/70 |
30 min |
2–12 |
24 |
||||||||
Humulin 20/80 |
30 min |
1–9.5 |
17–19 |
||||||||
Mixtard 20/80 |
30 min |
2–8 |
24 |
||||||||
Humulin 50/50 |
30 min |
2–12 |
16–18 |
||||||||
Mixtard 50/50 |
30 min |
4–8 |
24 |
||||||||
* Most preparations are available in a variety of presentations, including vials, cartridges for insulin pens, and pre-filled pens or devices. Humalog and Humulin preparations are manufactured by Eli Lilly, Lantus by Aventis, and all other listed preparations, including detemir, by Novo Nordisk. † None of the insulin analogues are registered for use in pregnancy. ‡ The long-acting analogues are not currently registered in Australia. § No defined peak of action. |
|||||||||||
5: Glycosylated haemoglobin (HbA1c) levels and diabetes complications
In normal glucose tolerance, HbA1c level is usually < 5%.
In diabetes, an HbA1c level > 8% indicates poor glycaemic control and a need to assess therapy and adherence.
A decrease in HbA1c level of 1 percentage point has been shown to lead to:
a 10% reduction in microvascular complications in type 2 diabetes (UKPDS trial13) and
a 20%–30% reduction in microvascular complications in type 1 diabetes (DCCT trial3), with associated less significant reductions in macrovascular complications.
In treating diabetes, the benefits of decreasing HbA1c level must be weighed against the risk of side effects and the ability of the patient to comply; thus, the “target” HbA1c level must be individualised.
HbA1c levels < 6% are very difficult to achieve in diabetes without serious side effects, such as life-threatening hypoglycaemia.

6: Case report — management as type 2 diabetes progresses
Presentation: A 51-year-old man with a 6-year history of type 2 diabetes presented to his general practitioner. He was an ex-smoker, with moderate alcohol consumption. He was taking metformin (500 mg twice daily) and was not prepared to consider changes in diet or exercise, despite considerable effort from the GP and a dietitian.
Examination and investigations: He weighed 102 kg, with body mass index 32 kg/m2 (reference range [RR], 20–25 kg/m2), and waist circumference, 110 cm (RR, < 94.0 cm). His glycosylated haemoglobin (HBA1c) level was 7.5%, reflecting good glycaemic control.
Management: The metformin dose was increased to 1 g twice daily. He again declined to modify diet or exercise. He was instructed to measure his fasting blood glucose level at least twice daily and to return for early review if readings were regularly over 8 mmol/L.
2-year review: He remained compliant with metformin therapy, but his HBA1c level had risen to 10%, and his blood pressure was 160/100 mmHg. Lipid levels were within the reference ranges. He still refused to modify diet or exercise. He had not been measuring blood glucose levels.
Management: The GP reiterated the importance of monitoring fasting blood glucose levels at home. A sulfonylurea and an angiotensin-converting enzyme (ACE) inhibitor were added to the regimen; metformin was continued.
3-year review: The HbA1c level had decreased to 7.8%, and blood pressure to 130/80 mmHg, but the patient had gained weight (110 kg). He was taking the maximum tolerated dose of metformin (1 g twice daily) and the maximum dose of the sulfonylurea.
Management: Diet and exercise changes were again suggested, with no success.
4-year review: The HbA1c level had risen to 8.9%.
Management: Insulin was judged to be necessary. Treatment choices were to:
Add intermediate-acting insulin at night (eg, Humulin NPH or Protaphane) at an initial dose of 10–20 U, to be titrated up based on early-morning blood glucose level, with continuation of sulfonylurea and metformin; or
Cease sulfonylurea, continue metformin and introduce insulin either twice daily (possibly using premixed insulin, including analogue mixtures) or four times daily (with regimens similar to those for type 1 diabetes); total daily insulin dose is often around 1 U/kg.
This patient demonstrates the need to change management as disease progresses.
Metformin was the initial drug of choice, as the major abnormality at that time was insulin resistance.
An alternative second-line therapy to a sulfonylurea would be a thiazolidinedione, which would have the advantage of further targeting the insulin resistance, but, currently in Australia, the choice is limited by prescribing restrictions and cost.
If metabolic control is suboptimal, introduction of insulin should not be delayed.
- 1. American Diabetes Association. Implications of the Diabetes Control and Complications Trial (position statement). Diabetes Care 2003; 26: 25-27.
- 2. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977-986.
- 3. White NH, Cleary PA, Dahms W, et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after conclusion of the Diabetes Control and Complications Trial (DCCT). Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications. J Pediatr 2001; 139: 1923-1928.
- 4. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima–media thickness in type 1 diabetes mellitus. N Engl J Med 2003; 348: 2294-2303.
- 5. Hoey H, Aanstoot HJ, Chiraelli F. Good metabolic control is associated with better quality of life in 2101 adolescents with type 1 diabetes. Diabetes Care 2001; 24: 1923-1928.
- 6. Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine reponses to hypoglycaemia during sleep. N Engl J Med 1998; 338: 1057-1062.
- 7. Wang F, Carabino JM, Vergara CM. Insulin glargine: a systematic review of long acting insulin analogue. Clin Ther 2003; 259: 1541-1577.
- 8. King AB, Armstrong D. A comparison of basal insulin delivery. Diabetes Care 2003; 26: 1322.
- 9. Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003; 2694: 1079-1087.
- 10. Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycaemia with long term continuous subcutaneous insulin infusion in type 1 diabetes. Diabetes Care 1996; 19: 324-327.
- 11. Ludvigsson J, Haas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients. Pediatrics 2003; 111: 933-938.
- 12. Chase HP, Kim LM, Owen SL, et al. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics 2001; 107: 222-226.
- 13. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405-412.
- 14. Craig ME, Handelsman P, Donaghue KC, et al. Predictors of glycaemic control and hypoglycaemia in children and adolescents with type 1 diabetes from NSW and the ACT. Med J Aust 2002; 177: 235-238.
- 15. Grey EA, Boland M, Davidson J, et al. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr 2000; 137: 107-113.
- 16. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003; 26: 2261-2267.
- 17. Hamilton J, Cummings E, Zdravkovic V, et al. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care 2003; 26: 138-143.
- 18. Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care 2001; 24: 643-645.
- 19. Shaw JE, Chisholm DJ. 1: Epidemiology and prevention of type 2 diabetes and the metabolic syndrome. Med J Aust 2003; 179: 379-383. <eMJA full text>
- 20. O’Moore-Sullivan TM, Prins JB. Thiazolidinediones and type 2 diabetes: new drugs for an old disease. Med J Aust 2002; 176: 381-386. <eMJA full text>
- 21. Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc 2003; 78: 411-413.
- 22. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003; 289: 2254-2264.
- 23. Scheen AJ. Current management strategies for coexisting diabetes mellitus and obesity. Drugs 2003; 63: 1165-1184.
- 24. Ahren B. Gut peptides and type 2 diabetes mellitus treatment. Curr Diab Rep 2003; 3: 365-372.






Abstract
As suboptimal blood glucose control has a lasting harmful effect even if control improves later, intensive insulin therapy to minimise hyperglycaemia is now recommended for all patients with type 1 diabetes.
The new rapid- and long-acting insulin analogues offer more physiological insulin profiles than traditional insulin preparations.
Continuous insulin infusion (“pump therapy”) may provide a solution for some patients with frequent hypoglycaemia or hypoglycaemic unawareness.
Continuous blood glucose monitoring reveals postprandial hyperglycaemia and asymptomatic nocturnal hypoglycaemia and may be especially useful for programming overnight basal insulin rates for pump therapy.
In type 2 diabetes, management should change with disease progression; introduction of insulin should not be delayed if metabolic control becomes suboptimal.
More individualised and physiological therapy is now possible