Needlestick injuries pose a potential occupational risk to healthcare workers, particularly through transmission of bloodborne viruses, such as hepatitis B and C viruses and HIV.1 An effective vaccine is available to prevent hepatitis B virus (HBV) infection,2 and post-exposure antiviral prophylaxis is effective in reducing HIV transmission.1,3 However, currently there are no measures that reduce hepatitis C virus (HCV) transmission.4 This is a potential problem given the growing epidemic of HCV infection in the Australian community and among hospital patients.5,6 Early recognition of acute HCV infection in healthcare workers may also reduce the risk of staff-to-patient transmission, as recent data suggest that early antiviral treatment of acute HCV infection vastly increases viral clearance.7
After a recent incident at our hospital in which a healthcare worker acquired HCV from a needlestick injury, we investigated the frequency of HCV exposure among healthcare workers at major metropolitan hospitals in Victoria and reviewed available guidelines for managing occupational exposures to HCV. We propose a risk-stratified standard protocol for follow-up of healthcare workers who suffer occupational needlestick injuries from source patients known to be HCV-infected.
The reported risk of HCV transmission after needlestick injury from an HCV-infected patient is considered to be 1.8%–3%.8-13 The transmission risk is predominantly from patients with hepatitis C viraemia detectable by polymerase chain reaction (PCR).8 The lower limit of HCV detection by PCR under optimal processing and storage conditions is generally 100 virus copies/mL, but variability in hepatitis C viraemia among some chronic HCV carriers means that PCR detection can fluctuate, even among those considered to be continuously viraemic.14,15 Perinatal maternal–fetal transmission, although not necessarily analogous to needlestick-injury transmission, appears more likely when maternal viral titres are above 106 copies/mL and is rare when mothers are PCR-negative.16,17
Current third-generation enzyme immunoassays for HCV antibody have nearly 100% sensitivity and detect seroconversion 4–10 weeks after infection. Delayed antibody detection and even false-negative results can occur among recently infected patients with renal failure, HIV infection and HCV-associated mixed essential cryoglobulinaemia.14 Elevation of serum alanine aminotransferase (ALT) concentration occurs after 4–12 weeks. Although used by some as a marker of recent HCV infection, raised ALT levels are not specific to this infection, and may fluctuate.14,18 In contrast, HCV viraemia can be detected by PCR between 10 days and 6 weeks after infection.19 Thus, PCR detection of hepatitis C viraemia appears more useful than HCV antibody or ALT testing in the early detection of acute HCV hepatitis.4,20 The Medicare schedule fee for HCV PCR is currently $90, compared with $15.30 for HCV antibody determination and $16.35 for liver function tests.21
We collaborated with six major metropolitan hospitals in Melbourne, Victoria, to determine the number of occupational exposures to blood and body fluids (including needlestick injuries) reported at each institution, and the number of these that involved a source patient with HCV infection as defined by the presence of HCV antibody. Consent was obtained from the source patient before testing for HCV (as well as HIV and HBV). Reported occupational exposures of healthcare workers to HCV at these hospitals is shown in Box 1. Of 1450 reported occupational exposures over 111 months, 138 were from HCV-infected patients. At all institutions, needlestick injuries made up more than 60% of the total number of exposures. There is currently no national Australian standard for the reporting of blood and body fluid exposures. Information was not available from all institutions on the rate of HCV among all inpatients, but at Hospital 1 the overall rate was 1.5% in 2002 (unpublished data), suggesting a possible reporting bias (approximately tenfold) towards exposures from HCV-positive source patients.
As information on the number and working hours of healthcare workers potentially exposed to needlestick injuries at each institution was not available, a healthcare worker- and time-dependent rate of needlestick injury could not be calculated. Given that our survey included only larger Melbourne hospitals, and that the predicted risk of HCV acquisition associated with each needlestick injury is 1.8%–3%,8-13 two to five healthcare workers are likely to be infected with HCV through occupational exposure in Victoria each year.
Acute HCV infection is symptomatic in 15%–20% of patients, but is rarely severe enough to require hospitalisation.20 However, about 75%–85% of HCV-infected patients develop chronic hepatitis, and about 15%–25% develop cirrhosis. Half of these individuals develop hepatic decompensation or hepatocellular carcinoma.14,22 Until recently, in the absence of evidence that any treatment was effective in preventing chronic hepatitis, early detection of acute HCV infection was considered to be of limited therapeutic importance. However, recent data show that early treatment with interferon-alfa monotherapy results in viral clearance in over 90% of recipients.7 Results for combined interferon and ribavirin in acute treatment of hepatitis C infection are currently inconclusive. The optimal timing of acute therapy after exposure remains uncertain, but some studies report similar results for immediate therapy and therapy that is delayed by 3–6 months.7,14,23-25
Many healthcare workers are extremely anxious after needlestick injuries, with widespread effects on work performance, personal relationships and psychological health, leading to depression and, at times, a sense of abandonment and isolation.26 This may occur regardless of counselling. Given the relatively low rate of HCV seroconversion after needlestick injury, it is often these psychological issues that have the greatest impact on injured healthcare workers. Anxiety can last for over a year, and the psychological counselling costs appear similar to the direct medical costs of these injuries.27,28 Many healthcare workers express concern about possible sexual transmission of HCV to partners, as well as maternal–fetal transmission for those who are currently pregnant or attempting to become pregnant. There is even a report of therapeutic abortion being sought.27 Although data on couples where one partner has chronic hepatitis C suggest that the rate of sexual transmission of HCV is low,29-32 the risk during seroconversion is less certain. Hence some HCV-exposed healthcare workers opt either to use condoms or to abstain from sexual contact until they are certain they have not acquired HCV,27 which may exacerbate interpersonal stress, depression and sense of social isolation. Early confirmation that HCV acquisition is unlikely could have positive effects on sexual and psychological health.
Ross and colleagues suggest that the risk of surgeons with known HCV infection (ie, HCV-positive on PCR) transmitting HCV to their patients is 1 in 1750 to 16 000 operations.33 However, the actual risk is likely to be influenced by factors such as viral load, the number and complexity of surgical procedures performed, and the surgeon’s technique and experience. It is often the less experienced junior medical staff who perform at-risk procedures after hours, when experienced supervision is least available, and fatigue is likely to be greatest. Fortunately, relatively few cases of HCV transmission from healthcare workers to patients have been reported, but such episodes have been associated with time-consuming and expensive “look-back” programs and considerable patient morbidity.34-37 Although there are few data, the risk of HCV transmission to patients is negligible among healthcare workers with no detectable HCV viraemia (ie, negative HCV PCR).8
Defining risk of transmission from healthcare workers may be helpful for legal as well as infection control reasons. There has been an instance where a surgeon had a work-related needlestick injury from an HCV-positive source patient at a major Australian hospital, and legal opinion obtained by the hospital’s administration was that the surgeon should cease all surgical procedures until confirmed as not having acquired HCV (Melbourne Infectious Diseases Group, personal communication, Jun 2001). This is contrary to recommendations from the US Centers for Disease Control and Prevention (CDC).1 A risk assessment structure regarding HCV transmission may have assisted decision-making in this case. Without it, management of needlestick injuries will become unworkable, as healthcare workers will become reluctant to report injuries involving HCV-infected patients if they believe they will be forced to cease clinical practice without any risk assessment or compensation.
A balance needs to be found between the rights of the injured healthcare worker and those of the healthcare worker’s future patients. Hospital administrations need to feel confident that needlestick injury reporting is accurate and that healthcare workers who are exposed to HCV are not placing their patients at significant risk of HCV transmission. Investigations that promptly identify acute HCV infection (eg, HCV PCR) could assist in identifying healthcare workers who should be re-deployed from exposure-prone procedures, while allowing other injured healthcare workers to continue routine practice. HCV antibody and ALT levels detect acute infection later than PCR, and neither gives an accurate assessment of healthcare worker infectivity. Clearly, the appropriate management of needlestick injuries poses a new challenge to the healthcare sector, both in terms of reducing the overall risk of needlestick injury, the fair and reasonable management of injured staff and the protection of patients.
Although some Australian guidelines have been proposed, they lack practical applicability and have not been widely adopted.38-39 Thus, many hospitals have developed their own protocols, resulting in substantial variability and subsequent confusion (and anxiety) among injured healthcare workers as to which protocol is most appropriate. Overseas recommendations for testing healthcare workers exposed to HCV vary widely and have recently been revisited.1,4,9,20,40-42 While all are based on an assessment of the likelihood of HCV acquisition, few consider the benefits of early disease recognition in terms of the health of healthcare workers, transmission to patients or legal risk assessment.
An effective management plan for prevention and management of needlestick injuries in healthcare workers needs to be multifaceted. Our proposed plan to reduce needlestick injuries and their risk is shown in Box 2. For post-needlestick injury management, we believe there needs to be a consistent approach by all healthcare institutions that recognises the current therapeutic, personal and legal context of HCV management in Australia. We propose an investigation protocol that considers the likely risk of HCV transmission not only from the source patient but also from the healthcare worker to other patients should the healthcare worker be infected (Box 3).
We have classified healthcare workers according to whether their occupation involves exposure-prone procedures, defined as those with “potential for direct contact between the skin (usually finger or thumb) of the healthcare worker and sharp surgical instruments, needles, or sharp tissues (spicules of bone or teeth) in body cavities or in poorly visualised or confined body sites”.38 Based on this, healthcare workers with potentially high risk of HCV transmission — “high transmitter risk” — include surgeons, operating room nurses, intensive care staff, interventional radiologists and their assistants, and emergency department staff. We consider all other healthcare occupations to be “low transmitter risk”.4,43
After an occupational exposure, the healthcare worker should be counselled about the degree of risk associated with the type of exposure: needlestick injuries pose a greater risk than splashes, and those from a hollow-bore needle a greater risk than those from a solid needle.1,3,39 We also propose that the protocol considers the presence of HCV viraemia in the source patient, and includes early detailed assessment of HCV acquisition among healthcare workers in whom early disease recognition could have widespread consequences. These consequences may include significant psychological effects which, although possibly disproportionate to the transmission risk of the injury, will be more easily resolved by early evidence that HCV transmission is unlikely. Other aspects of the counselling process that can help alleviate healthcare worker anxiety include rapid initiation of testing, reminders about when to have follow-up testing or vaccination and, when possible, continuity of care at subsequent visits.27
Injured healthcare workers should remain on routine duties after needlestick injury unless there is evidence of HCV acquisition. The latter should be assessed, counselled and offered appropriate therapy by experts in HCV management and treatment.
Our suggested protocol is similar to the protocols of other groups, with several notable exceptions. The CDC recommends HCV antibody and ALT testing at baseline and 6 months after needlestick injury. PCR testing may be done at 4–6 weeks “if earlier diagnosis of HCV infection is desired”. These guidelines do not take into account source viraemia or the healthcare worker’s transmitter status.1 In fact, the CDC recommendations state that “health care professionals exposed to HBV- or HCV-infected blood do not need to take any special precautions to prevent secondary transmission during the follow-up period; however, they should refrain from donating blood, plasma, organs, tissue, or semen”. This statement, which is referenced to a previous CDC recommendation,4 is contradictory in terms of potential transmission risk. Sulkowski et al, who recently described a case similar to ours in which a healthcare worker was infected with HCV after a work-related needlestick injury, proposed a modified CDC investigation regimen for healthcare workers after injury, but this still did not stratify according to transmission risk of either source or healthcare worker.20 Previous Australian guidelines recommend HCV antibody testing only at 0 and 3 months.39 The British and Canadian protocols are also not specific.40-42
We believe that our proposed post-needlestick injury HCV investigation protocol provides a practical approach for assessing injured healthcare workers in the current therapeutic and legal contexts of HCV management in Australia. We encourage the development and acceptance of a common protocol for use in all Australian hospitals.
1: Blood and body fluid exposures among healthcare workers at major metropolitan hospitals in Victoria
Hospital |
Months of assessment (dates) |
Blood and body-fluid exposures† |
HCV exposures* |
||||||||
Total (% of all exposures) |
Annualised no. |
||||||||||
1 |
29 (1/99–5/01) |
335 |
36 (11%) |
15 |
|||||||
2 |
16 (1/00–4/01) |
205 |
26 (13%) |
19 |
|||||||
3 |
5 (1/01–5/01) |
34 |
6 (18%) |
14 |
|||||||
4 |
24 (1/99–12/00) |
468 |
25 (5%) |
12 |
|||||||
5 |
29 (1/99–5/01) |
363 |
40 (11%) |
17 |
|||||||
6 |
8 (4/00–11/00) |
45 |
5 (11%) |
7 |
|||||||
Total |
111 |
1450 |
138 (9.9%) |
84 |
|||||||
* Patient was positive for antibodies to hepatitis C virus. † 60%–85% were needlestick injuries. |
|||||||||||
2: Proposed management plan to reduce needlestick injuries and their risk among healthcare workers
1. Reduction in the risk of needlestick injuries and other exposures through:
Adequate education of healthcare workers about phlebotomy and intravenous cannula insertion, with credentialling of knowledge and performance.
Systems management
Availability of suitable sharps-disposal containers
Introduction of safety cannulas
Rationalisation/avoidance of unnecessary procedures
Appropriate healthcare worker workload and adequate staff–patient ratios (excessive tiredness and work-related stress are clearly associated with higher rates of needlestick injury).
2. Appropriate health management and follow-up systems for staff, including appropriate counselling about hepatitis B and C virus and HIV infection.
3. Appropriate vaccination program for healthcare workers, especially hepatitis B vaccination, to prevent bloodborne diseases.
3: Proposed protocol for follow-up of healthcare workers after needlestick injury involving a patient with hepatitis C virus infection
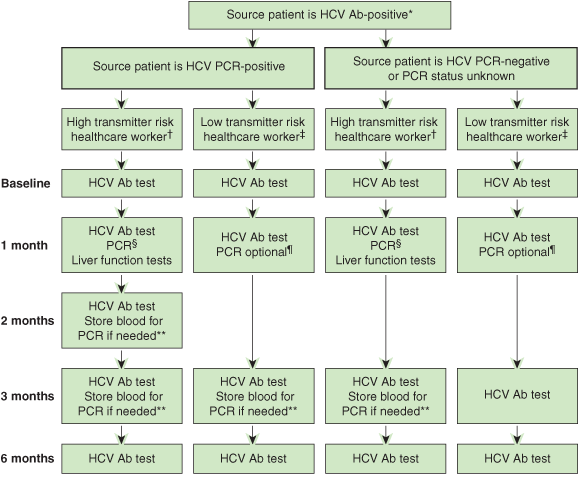
HCV Ab = antibody to hepatitis C virus. PCR = polymerase chain reaction.
* Renal dialysis patients may have false-negative HCV Ab results and should be screened for HCV infection with both HCV Ab and PCR testing.
† “High transmitter risk” healthcare workers comprise surgeons, operating room nurses, interventional radiologists and their assistants, and emergency department and intensive care staff.
‡ “Low transmitter risk” healthcare workers comprise all others.
§ PCR done to allow early treatment if infected, to assess potential for healthcare worker-to-patient transmission and for legal reasons.
¶ PCR done for healthcare worker mental health and sexual advice.
** For possible retrospective PCR if HCV Ab seroconversion occurs.
- Patrick GP Charles1
- M Lindsay Grayson2
- Peter W Angus3
- Joseph J Sasadeusz4
- 1 Department of Infectious Diseases, Austin and Repatriation Medical Centre, Heidelberg, VIC.
- 2 Department of Gastroenterology, Austin and Repatriation Medical Centre, Heidelberg, VIC.
- 3 Victorian Infectious Diseases Service, Royal Melbourne Hospital, Parkville, VIC.
Many of the proposals in this protocol are the result of a meeting of clinicians held in Melbourne, Victoria, in 2001 to discuss a standardised approach to nosocomial HCV transmission. We acknowledge the input of these clincians and also the assistance of the infection control practitioners who helped obtain data: Ms Rhea Martin (Austin and Repatriation Medical Centre), Ms Fiona Wilson (Western General Hospital), Ms Joanne Cocks (St Vincent’s Hospital), Mr Richard Bartolo (Mercy Hospital), Associate Professor Denis Spelman (Alfred Hospital) and Dr Alan Street (Royal Melbourne Hospital).
None identified.
- 1. Centers for Disease Control and Prevention. Updated US Public Health Service Guidelines on the management of occupational exposures to HBV, HCV, and HIV and recommendations for post-exposure prophylaxis. MMWR Recomm Rep 2001; 50 (RR-11): 1-67.
- 2. National Health and Medical Research Council. Australian immunisation handbook. 7th ed. Canberra: AGPS, 2000.
- 3. Case–control study of HIV seroconversion in health-care workers after percutaneous exposure to HIV-infected blood — France, United Kingdom, and United States, January 1988–August 1994. MMWR Morb Mortal Wkly Rep 1995; 44: 929-933.
- 4. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998; 47 (RR-19): 1-39.
- 5. Batey RG. Hepatitis C: where are we at and where are we going? Med J Aust 2002; 176: 361-362. <eMJA full text>
- 6. Watson KJR. Preventing hepatitis C virus transmission in Australians who inject drugs. Med J Aust 2000; 172: 55-56.
- 7. Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001; 345: 1452-1457.
- 8. Dore GJ, Kaldor JM, McCaughan GW. Systematic review of role of polymerase chain reaction in defining infectiousness among people infected with hepatitis C virus. BMJ 1997; 315: 333-341.
- 9. Recommendations for follow-up of health-care workers after occupational exposure to hepatitis C virus. MMWR Morb Mortal Wkly Rep 1997; 46: 603-606.
- 10. Hamid SS, Farooqui B, Rizvi Q, et al. Risk of transmission and features of hepatitis C after needlestick injuries. Infect Control Hosp Epidemiol 1999; 20: 63-64.
- 11. Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16: 1109-1114.
- 12. Osborn EHS, Papadakis MA, Gerberding JL. Occupational exposures to body fluids among medical students: a seven-year longitudinal study. Ann Intern Med 1999; 130: 45-51.
- 13. Torres M, Campins M, Esteban JI, et al. Is it useful to perform the RNA test for hepatitis C in health care workers after an accidental needlestick? J Hepatol 2000; 33: 686.
- 14. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345: 41-52.
- 15. Beld M, Penning M, McMorrow M, et al. Different hepatitis C virus (HCV) RNA load profiles following seroconversion among injecting drug users without correlation with HCV genotype and serum alanine aminotransferase levels. J Clin Microbiol 1998; 36: 872-877.
- 16. Terrault NA. Epidemiological evidence for perinatal transmission of hepatitis C virus. Viral Hepatitis Reviews 1998; 4: 245-258.
- 17. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. N Engl J Med 1994; 330: 744-750.
- 18. National Institutes of Health. National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: 2002–Jun 10-12, 2002. Hepatology 2002; 36 (5 Suppl 1): S3-S20.
- 19. Zaaijer HL, Cuypers HT, Reesink HW, et al. Reliability of polymerase chain reaction for detection of hepatitis C virus infection. Lancet 1993; 341: 722-724.
- 20. Sulkowski MS, Ray SC, Thomas DL. Needlestick transmission of hepatitis C. JAMA 2002; 287: 2406-2413.
- 21. Australian Department of Health and Ageing. Medicare Benefits Schedule, November 2002. Canberra: Commonwealth of Australia, 2002.
- 22. Seeff LB. Natural history of hepatitis C. Hepatology 1997; 26 (3 Suppl): 21S-28S.
- 23. Quin JW. Interferon therapy for acute hepatitis C viral infection — a review by meta-analysis. Aust N Z J Med 1997; 27: 611-618.
- 24. Poynard T, Leroy V, Cohard M, et al. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996; 24: 778-789.
- 25. Vogel W. Treatment of acute hepatitis C virus infection. J Hepatol 1999; 31 Suppl 1: 189-192.
- 26. Rosenstock L. Needlestick injuries among healthcare workers. Statement of Linda Rosenstock, MD, MPH, Director, CDC's National Institute for Occupational Safety and Health, before the Committee on Education and the Workforce Subcommittee on Workforce Protections, US House of Representatives, June 22, 2000. Available at: http://www.cdc.gov/washington/testimony/ws062200.htm (accessed Jun 2003).
- 27. Gershon RRM, Flanagan PA, Karkashian C, et al. Health care workers’ experience with postexposure management of bloodborne pathogen exposures: a pilot study. Am J Infect Control 2000; 28: 421-428.
- 28. Fisman DN, Mittleman MA, Sorock GS, Harris AD. Willingness to pay to avoid sharps-related injuries: a study in injured health care workers. Am J Infect Control 2002; 30: 283-287.
- 29. Kao JH, Hwang YT, Chen PJ, et al. Transmission of hepatitis C virus between spouses: the important role of exposure duration. Am J Gastroenterol 1996; 91: 2087-2090.
- 30. Goto M, Fujiyama S, Kawano S, et al. Intrafamilial transmission of hepatitis C virus. J Gastroenterol Hepatol 1994; 9: 13-18.
- 31. Caporaso N, Ascione A, Stroffolini T. Spread of hepatitis C virus infection within families. Investigators of an Italian Multicenter Group. J Viral Hepat 1998; 5: 67-72.
- 32. Guadagnino V, Stroffolini T, Foca A, et al. Hepatitis C infection in family setting. Eur J Epidemiol 1998; 14: 229-232.
- 33. Ross RS, Viazov S, Roggendorf M. Risk of hepatitis C transmission from infected medical staff to patients: model-based calculations for surgical settings. Arch Intern Med 2000; 160: 2313-2316.
- 34. Ross RS, Viazov S, Gross T, et al. Transmission of hepatitis C virus from a patient to an anesthesiology assistant to five patients. N Engl J Med 2001; 343: 1851-1854.
- 35. Esteban JI, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med 1996; 334: 555-560.
- 36. Duckworth GJ, Heptonstall J, Aitken C. Transmission of hepatitis C virus from a surgeon to a patient. Commun Dis Public Health 1999; 2: 188-192.
- 37. Brown P. Surgeon infects patient with hepatitis C. BMJ 1999; 319: 1219.
- 38. National Health and Medical Research Council. Infection control in the health care setting. Guidelines for the prevention of transmission of infectious diseases. Canberra: AGPS, 1998.
- 39. Australian National Council on AIDS. Bulletin No. 16. Needlestick and blood accidents. Canberra: AGPS, 1996.
- 40. Ramsay ME. Guidance on the investigation and management of occupational exposure to hepatitis C. Commun Dis Public Health 1999; 2: 258-262.
- 41. Health Canada. An integrated protocol to manage health care workers exposed to bloodborne pathogens. Can Commun Dis Rep 1997; 23 Suppl 2: 1-14.
- 42. Sherman M. Management of viral hepatitis: Clinical and public health perspectives — a consensus statement. Can J Gastroenterol 1997; 11: 407-416.
- 43. Polish LB, Tong MJ, Co RL, et al. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control 1993; 21: 196-200.





Abstract
The increasing rate of hepatitis C virus (HCV) infection in the community means that there is increased risk of occupational exposure for healthcare workers.
In metropolitan hospitals in Victoria, we found that 80–150 healthcare workers have occupational exposures from HCV-infected patients annually.
As there is a 1.8%–3% risk of transmission of HCV from a needlestick injury, two to five healthcare workers are likely to acquire HCV each year in Victoria.
These needlestick injuries pose a personal, legal and professional risk to healthcare workers and their patients.
Recent information shows that early antiviral treatment of acute HCV infection has high cure rates.
Current local and international protocols for management of healthcare workers exposed to HCV do not address these issues.
We propose a management protocol after needlestick injury that is stratified according to the likelihood of HCV acquisition and potential risk of staff-to-patient transmission, and that is consistent with the current legal and clinical context of HCV infection in Australia.