Cervical screening in Australia is one of the great public health success stories, as witnessed by a continuing dramatic fall in the incidence of carcinoma of the cervix and mortality from this disease (Box) since the introduction of the National Cervical Screening Program (NCSP).1,2 Here, we highlight two areas of current interest — quality assurance and cost control.
A critical aspect of the NCSP is that screening tests are performed at an optimal level.3 Asymptomatic women and their health carers who participate in screening assume that this is the case.
Since 1987, Australia has had compulsory laboratory accreditation. The National Pathology Accreditation Advisory Committee (NPAAC) sets quality standards for patho-logy laboratories, and The National Association of Testing Authorities and the Royal College of Pathologists of Australasia inspect individual laboratories triennially to ascertain standards compliance.
For gynaecological cytology, these quality standards include performance standards, which are numerical outcome measures set by the NCSP, in conjunction with the NPAAC. Although the performance standards comprise only one part of an inspection, if a laboratory falls outside these standards this may indicate that the overall reporting quality is substandard.
A recent independent audit of the NCSP highlighted that the standards which had been set were valid and the inspection process was sound.4 However, the 3-year interval between inspections and the lengthy appeal process meant that Medicare was continuing to pay for smears from laboratories with questionable quality for excessive periods of time. In response to this audit, a number of initiatives have been undertaken or are currently in progress.
The annual cost of the NCSP to government in 1993 (the most recent evaluation) was $138 million.6 Cervical screening is expensive when expressed in a league table of cost per quality-adjusted life-year saved.7
In the program, the main pressure points for cost control include: participation, the proportion of women requiring further investigation, and new technologies.
Australia has a 2-year screening interval.8 However, there are no barriers to women being screened more frequently than this, and all such tests are reimbursed by Medicare. Early rescreening is exceedingly common: almost half the women with negative Pap smear reports in 1997 had a further smear before 24 months had elapsed.1
The 2-year screening policy is under review. It is a short rescreening interval by international standards.8 Alternatively, significant cost savings could be achieved if Medicare were to pay for only one screening test every 2 years. We estimate that the potential savings to government from reduced claims to Medicare could be of the order of $32 million per year (calculations available on request).
The lifetime risk of a woman being diagnosed with cervical cancer in a developed country like Australia, in the absence of any screening, has been estimated as 1.58%.9 By contrast, the lifetime risk of a woman having a colposcopy in Australia is 76.8%.10 These figures suggest significant overinvestigation, with most investigated abnormalities having a very low probability of becoming malignant.
With recent advances in understanding of the natural history of cervical abnormalities (particularly the transient nature of most low-grade abnormalities), and the improved quality of cytology reporting, it is timely to review current recommendations for managing women with screen-detected abnormalities. This review is in progress.11
Mortality from cervical cancer in Australia
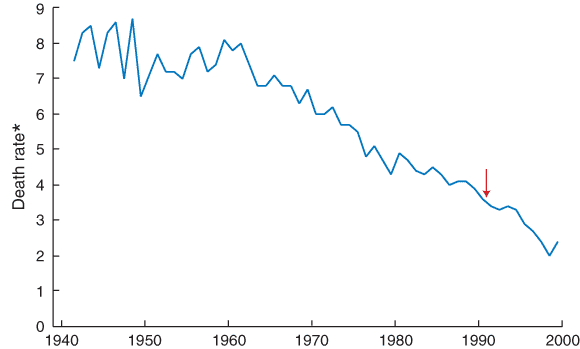
*Age-standardised death rate (Australian Standard Population 1991).
Cervical screening has been available for Australian women since the 1960s.1 The arrow shows when the organised approach to cervical screening commenced (1991). (Redrawn from: Australian Institute of Health and Welfare. Australian long term trends in mortality. Canberra: AIHW, 2002.)
- 1. Australian Institute of Health and Welfare (AIHW). Cervical screening in Australia 1997-1998. AIHW Catalogue No. 9. Canberra: AIHW, 2000: 36. Available at: http: //www.aihw.gov.au/publications/can/csa97-8/csa97-8.pdf (accessed May 2003).
- 2. A decade of change. A report on Australia's National Cervical Screening Program 1989-1999. Canberra: Commonwealth Department of Health and Aged Care, 2000.
- 3. Report of the Steering Group on Quality Assurance in Screening for the Prevention of Cancer of the Cervix. Making the Pap smear better. Canberra: AGPS, 1993.
- 4. Australian National Audit Office. The National Cervical Screening Program. Audit Report No. 50 2000-2001. Canberra: Australian National Audit Office, 2001.
- 5. Health Access and Financing, Department of Health and Ageing. Report of the evaluation of the pathology laboratory accreditation arrangements, 2002. Available at: http: //www.health.gov.au/haf/branch/dtb/evaluation.htm (accessed May 2003).
- 6. Report of the Evaluation Steering Committee. The interim evaluation of the organised approach to preventing cancer of the cervix 1991-95. Canberra: AGPS, 1995: 27. Available at: http: //www.health.gov.au/pubhlth/publicat/document/eval.pdf (accessed May 2003).
- 7. Australian Health Ministers' Advisory Council. Cervical Cancer Screening Evaluation Committee. Cervical cancer screening in Australia: options for change. Australian Institute of Health: Prevention Program Evaluation Series No 2. Canberra: AGPS, 1991: 90.
- 8. Dickinson JA. Cervical screening: time to change the policy. Med J Aust 2002; 176: 547-550. <MJA full text>
- 9. IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. BMJ 1986; 293: 659-664.
- 10. Kavanagh AM, Santow G, Mitchell H. Consequences of current patterns of Pap smear and colposcopy use. J Med Screen 1996; 3: 29-34.
- 11. National Cervical Screening Program Guidelines Review Group. Available at: www.csp.nsw.gov.au/nhmrc (accessed May 2003).





Abstract
Cervical screening in Australia is a successful public health initiative.
Since the introduction of the National Cervical Screening Program in 1991, there has been a significant fall in incidence of and mortality from cervical cancer.
Laboratory quality procedures are critical to ensuring optimal outcomes.
Laboratory accreditation procedures are being reviewed in line with recent government recommendations.
For a sustainable program, cost-containment issues need to be considered; screening interval, management of screen-detected abnormalities, and new technologies are the critical drivers of cost.