The increasing number of medications available is a significant contributor to the burgeoning expenditure on prescription drugs in Australia1 and internationally.2
Once listed in the Australian Pharmaceutical Benefits Schedule (PBS), a medication may be prescribed under one of three categories: unrestricted, restricted (for specific therapeutic uses only) and authority required (requiring prior approval from the Health Insurance Commission). Of the 1466 drugs on the current list, 297 require an authority prescription.3
In 1991 the Pharmaceutical Health and Rational Use of Medicines Working Party (PHARM) was formed to examine the rational use of medicines and drug policy objectives with respect to health outcomes.4 In 1992, the Federal Government introduced the Quality Use of Medicines (QUM) initiative — an important element of the Australian National Medicines Policy — which aimed to address widespread concerns about the health, social and economic costs to Australia associated with the inappropriate use of prescribed and over-the-counter medications.5 QUM has a regulatory, managerial and an educational dimension.
A 1992 parliamentary inquiry into drug prescribing and supply6 noted that the Authority Prescribing System (APS) "is a crude form of cost containment and not an efficient way of providing universal access to drugs for the general community". The process of seeking and being provided with the authority to prescribe certain, usually expensive, drugs has evolved over the years. However, because it appears to reduce uptake of expensive medications, "authority required" prescribing has remained a core strategy in the APS.
The University of Melbourne Human Research Ethics Committee approved the study, which comprised a questionnaire survey of medical practitioners seeking demographic information and responses to 14 statements about the APS and to eight vignettes on authority prescribing issues. A previous qualitative phase of interviews and focus groups, involving 50 doctors, identified doctors' key concerns about the APS and informed the development and piloting of the questionnaire.
Case vignettes in the questionnaire were developed to carefully vary according to eight situational factors, which have certain given "levels" (in brackets). These were:
source of prescription (doctor/other doctor/patient);
cost (pensioner/private);
privacy concern (yes/no);
authority indicators (present/absent);
credible evidence for using authority drug (present/absent);
indication for drug (present/absent);
request for increased amounts of drugs (yes/no); and
familiarity with medication (yes/no).
An example of these vignettes is provided in Box 1.
For each vignette, doctors were questioned on five aspects of concern about the APS. These were how much they agreed that the use of the drug:
was appropriate;
was in the best interests of the patient;
was ethical;
was evidence-based; and
influenced the doctor–patient relationship.
They rated their responses along a five-point Likert scale (1 = strongly agree to 5 = strongly disagree). Such vignettes are known as factorial vignettes, designed to assess how much influence a specific situational factor has in a given aspect of the domain being studied.7,8
The 40-year cut-off was chosen as it is the age used by the Royal Australian College of General Practitioners in defining its Young Practitioners Group (see http://www.racgp.org.au) and was used to categorise the sample by age. Pearson's χ2 statistic was used to compare demographic characteristics (provided by the HIC) of responders with non-responders and computerised prescribers with non-computerised prescribers.
Five of the 14 statements on the APS, representing the socioeconomic rationale, quality, safety, privacy and computerisation aspects, were selected for presentation in Box 2. Because it was strongly associated with doctors' age and practice type, analysis for "computers made authority prescribing easier" was stratified by computerised prescribing.
A hierarchical model was fitted to each of the five aspects of concern about the APS, where the doctors' responses were assumed to be random and the eight situational factors fixed for each vignette. Familiarity with medication was dropped from the model because it was linearly dependent on the other factors. The coefficients from the model estimated the degree of change in agreement with the statements (ie, the "difference") when the situational factor was present compared with when it was absent. Thus, the difference measures how much importance doctors placed on the situational factors across the five aspects of concern about the APS. Because the sample in this study was large, all the situational factors were statistically significant, even though some of the differences were not of practical value. By convention, differences were considered to be of practical value when the absolute difference is ≥ 0.4, and highly significant when > 0.1. A positive difference denoted disagreement and a negative difference denoted agreement. Data were analysed in STATA.9
Over half the doctors used a computer to prescribe (53%) or had email (57%) and Internet access (58%) in their offices. Computerised prescribing was significantly associated with age (68% ≤ 40 years v 48% > 40 years; P < 0.001), practice type (70% GPs v 10% specialists; P < 0.001) and number of prescriptions (23% for 0–4, 43% for 5–9 and 59% with 10 or more prescriptions in three months; P < 0.001). No urban and rural differences in computerised prescribing (51% v 57%; P = 0.19) were detected.
Box 2 shows the doctors' perceptions of the APS. Most believed there was a socioeconomic rationale for the APS, but disagreed that the APS reduced the frequency of adverse drug reactions. Forty-three per cent (132/306) of the doctors who did not use computers for prescribing answered "don't know" or did not give a response to the statement about computerisation, compared with less than 2% of those who did use computers. Of the remaining 529 doctors who responded to the computerisation statement, doctors who used computers were more likely to agree that computerised prescribing made authority prescribing easier than those who did not use computers (70% v 25%; P < 0.001).
Box 3 summarises the relationship between the five aspects of concern to doctors regarding the APS and seven situational factors. Doctors perceived positive effects on all five aspects if the patient's regular doctor initiated the prescription process instead of another doctor or the patient, and negative effects when credible evidence supported the APS indication for the medication or when increased amounts of medication were requested. Adherence to APS criteria was considered to be appropriate use of the APS and an ethical way to deal with the patient's problem; however, it was not thought to positively influence the doctor–patient relationship. Where a clinical indication was present, even if the indication or patient did not fit APS criteria, doctors believed that prescribing an APS medication was in the patient's best interest, positively influenced the doctor–patient relationship, that it was an ethical way to deal with the patient's problem, and that it conformed to the best available evidence. Privacy concerns, and perhaps costs incurred by patients, were seen as a negative influence on the doctor–patient relationship.
GP characteristics, such as rural or metropolitan, high or low prescriber, and generalist or specialist, were not significant factors in the model adopted (data not shown).
Stratification and oversampling were reasons for the significantly lower response rate from doctors in metropolitan and large rural areas. Low prescribers were significantly under-represented, perhaps reflecting the less strong opinions or concerns of this group. Otherwise, the sample is representative of doctors who prescribed an authority medication at least four times in the past three months in Australia.
While our survey was carefully constructed to be realistic, with high reliability and internal validity, it is important to note that any self-report instrument can measure only decision-making and may not predict actual behaviour in similar situations.
The positive perception about the socioeconomic, access and equity aspects of the APS demonstrates doctors' appreciation that the APS is a regulatory program to bring the most medications to the most people at the least cost. There was significant concern about how the telephone authority approval process may compromise patient privacy.
The relatively high levels of computer use, particularly for prescribing in general practice, and the recent successful demonstration of an Internet-based "Authority Notification system,"10 augur well for the use of information and communication technologies to improve APS processes and redress privacy and information security concerns.
Doctors' responses to the factorial vignettes demonstrated a fundamental difference between the APS criteria and the attitudes to authority prescribing. Doctors placed greater emphasis on the influence of the doctor–patient relationship, clinical needs and benefits to patients than on the APS criteria in making prescribing decisions. This patient-centred practice seems to be fundamental to why medications are sometimes prescribed outside of PBS/APS approved uses.
The attitudes reported by doctors in this study appear to support the assertion by the 1992 Parliamentary Inquiry into the Prescription and Supply of Drugs6 that authority-required prescribing may not be achieving the stated aims of the National Medicines Policy to reduce variations in prescribing by combining evidence, the need for rationing, and education on quality prescribing.
To avoid alienating the health profession, the process of setting criteria for prescribing a medication in the APS must be transparent and acknowledge the socioeconomic rationale of the APS. It must be recognised that, while doctors appreciated the socioeconomic rationale for the APS in making prescribing decisions, the documented and approved use of a medication is tempered by clinical and patient considerations. The professional and ethical conundrum associated with prescribing outside of PBS/APS approved use for clinical and patient-centred reasons must be addressed. Strategies should build on this fundamental patient-centredness of the profession to promote appropriate prescribing and use of medicines, rather than more bureaucratic processes to restrict prescribing. Evidence-based education programs for doctors and patients, within a context of notification of medicines prescribed and clinical indications, may be more cost-effective than restricted prescribing.
1: Example of a case vignette used in the questionnaire
Case scenario 4
A 45-year-old male, on unemployment benefits, had been seeing his doctor for epigastric pain for six weeks. The pain was worse after meals and not relieved by Zantac. An endoscopy demonstrated a small hiatus hernia, with grade 2 inflammation and no ulceration. A helicobacter test was negative. The endoscopist had started the patient on Omeprazole, which relieved the patient's symptoms. The patient returned to the doctor and requested a prescription for Omeprazole. The doctor was confident using Omeprazole, and prescribed it on authority, citing "severe ulcerating oesophagitis".
Information from Schedule of Pharmaceutical Benefits (effective from 1/11/2000) Omeprazole (Losec, Acimax p.72): Severe refractory ulcerating oesophagitis proven by endoscopy.
2: Doctors' opinions relating to the Authority Prescribing System (APS) (n = 669)*
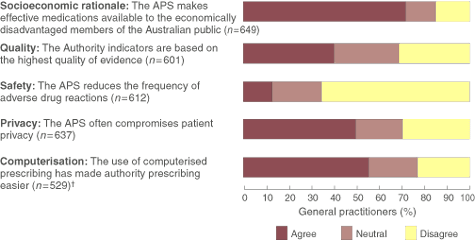
* Reduced total number of responders because of doctors responding "Don't know" or not responding. † 132/138 (96%) respondents who answered "Don't know" or did not give a response to the statement about computerisation did not use a computer to prescribe. Of the 529 who responded to the statement, 65% said they use a computer to prescribe, 33% said they did not use a computer to prescribe and 2% did not respond. Two respondents left both responses blank.
3: Associations of the seven situational factors with each aspect of the Authority Prescribing System
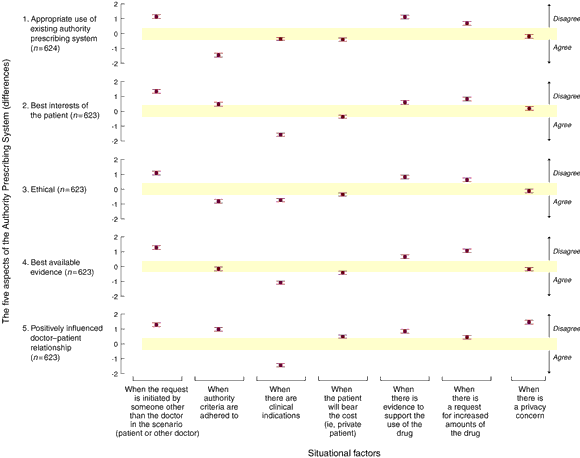
The plotted points represent differences and 95% confidence intervals. The differences are those between the mean score of the ratings if the factor on the x-axis was present and the mean score of the ratings if the factor was not present. By convention, an absolute "difference" is considered of practical value if it is greater than 0.4 and of high practical significance if greater than 1.0. Plotted differences that lie in the shaded area (between -0.4 and 0.4) in each of the five plots are considered not of practical significance. Differences that lie outside the shaded area are of practical significance. A positive difference denotes disagreement for the aspect if the situational factor was present and a negative difference denotes disagreement for the aspect if the factor was present.
Received 17 January 2002, accepted 17 July 2002
- Siaw-Teng Liaw1
- Christopher M Pearce2
- Patty Chondros3
- Leone Piggford4
- Kay Jones5
- Barry P McGrath6
- 1 Department of General Practice, The University of Melbourne, Carlton, VIC.
- 2 Department of Rural Health, The University of Melbourne, Shepparton, VIC.
The Quality Use of Medicines Evaluation Program of the Commonwealth Department of Health and Aged Care funded this study. We thank the Advisory Committee (Professors R Moulds, B Jackson and L Roller, Drs M Leviotidis, S Whicker, P MacIsaac and P MacManus), staff and associates of the University of Melbourne Department of General Practice, participants in the interviews, focus groups and teleconferences, and the respondents to the national survey.
None identified.
- 1. Health expenditure: its management and sources (Report No. 0 642 41563 3). Canberra: Commonwealth Department of Health and Aged Care, 1999.
- 2. Australian statistics on medicines 1998. Canberra: Commonwealth Department of Health and Aged Care, 1998.
- 3. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners (effective from 1 February 2001). Canberra: Commonwealth Department of Health and Aged Care, 2001.
- 4. Murray M. Australian national drug policies facilitating or fragmenting health? J Dag Hammarskjold Foundation 1995; 1: 148-192.
- 5. A policy on the quality use of medicines. Canberra: Commonwealth Department of Health, Housing and Community Services; 1992.
- 6. House of Representatives Standing Committee on Community Affairs. Prescribed health part 1: regulation and the pharmaceutical industry. (Report No. 0 644 24344 9.) Canberra: The Parliament of the Commonwealth of Australia, 1992.
- 7. Rossi P, Nock S, editors. Measuring social judgements: the factorial survey approach. Beverley Hills: Sage; 1982.
- 8. Rossi P, Anderson A. The factorial survey approach: an introduction. In: Rossi P, Nock S, editors. The factorial survey approach. Beverley Hills: Sage; 19815-68.
- 9. StataCorp. Stata Statistical Software: Release 7.0. College Station, Tx: Stata Corporation; 2001.
- 10. Watt M, Dawson S, McCallum S. The Authority Notification System (ANS) trial. In: McCormick H, McPhee W, editors. Proceedings of the RACGP 11th computer conference. Royal Australian College of General Practitioners, South Melbourne: 9-11, 2001.





Abstract
Objective: To examine doctors' perceptions and attitudes to prescribing within the Authority Prescribing System (APS).
Design and setting: Questionnaire survey of Australian doctors' responses to a number of statements and factorial vignettes, conducted between 1 May and 30 June 2001.
Participants: A national random sample of 1200 doctors, stratified according to specialist/generalist, rural/urban and high/low prescriber: 669 (56%) responded.
Main outcome measures: Self-reported perceptions of the APS and attitudes to prescribing within the APS.
Results: 72% of doctors agreed that the APS makes effective medications available to the socioeconomically disadvantaged members of the Australian public and 50% agreed that it compromises patient privacy. Fewer agreed that authority indicators were based on the highest quality of evidence quality (40%) or medication safety (12%). Doctors placed more emphasis on the doctor–patient relationship than on the criteria for authority prescribing in their decisions about prescribing APS medications. Doctors who used computers to prescribe were more likely to agree that computers can improve the authority prescribing process.
Conclusions: This study suggests that authority-required prescribing is not achieving the stated aims of the National Medicines Policy in reducing variability in prescribing. Strategies to improve the quality of prescribing must consider the professional and ethical conundrum associated with prescribing outside of PBS/APS approved use for clinical and patient-centred reasons.