The most widespread of all hookworm species, Ancylostoma caninum, parasitises dogs throughout most of the humid tropical and subtropical regions, including the mainland of Australia. In recent years, the realisation that A. caninum can cause human gut disease1,2 has sparked renewed interest in its biology and epidemiology. In north-eastern Australia, human eosinophilic enteritis has been diagnosed relatively frequently, and A. caninum infection accounts for most cases. Although only single worms have ever been recovered from patients, and egg production has never been detected,1,2 anthelmintic treatment leads to a rapid clinical response. Relapse of abdominal symptoms and blood eosinophilia in some patients is presumably a result of either reinfection, or reactivation of third-stage (the infective stage) larvae (L3) dormant in the tissues, which then undergo tracheal migration to reach the gut.1,2
The precise route of human infection has not been established. While opportunities for percutaneous exposure abound in the endemic regions, such as coastal Queensland, where dog faeces contaminate grass and soil and people frequently walk barefoot, the possibility of oral exposure has never been seriously considered or investigated.
In the normal canine host, infection with A. caninum usually follows skin contact with L3 that have developed in soil. Invasive L3 then undergo either tracheal migration to reach the gut (leading to obvious infection as early as 15 days after exposure), or somatic migration to invade tissue reservoirs (mainly skeletal muscle fibres), where they become dormant.2,3
While percutaneous exposure has been assumed to explain human infections with A. caninum, experimental or accidental inoculation via this route has uniformly failed to provoke abdominal symptoms, significant blood eosinophilia or antibody responses (P P, unpublished data).4 Single doses of 50 L3, although provoking itchy skin lesions, failed to generate detectable circulating white cell or antibody responses in one volunteer, while a man exposed to 100 L3 per month for 18 months maintained normal blood cell counts throughout, becoming weakly seropositive on western blot after five months and by ELISA (enzyme-linked immunosorbent assay) after 12 months. Neither volunteer experienced abdominal symptoms.
This study aimed to determine the effects of oral exposure to A. caninum larvae by observing symptomatic and haematological responses in an informed volunteer who ingested a number of L3 which we considered would be acquired under conditions of natural exposure.
Infective larvae of A. caninum were obtained by culturing faeces from naturally infected dogs (subsequently autopsied at the University of Queensland Veterinary School to confirm hookworm species). The procedure is shown in Box 1.
The human volunteer (J K L) was a fully-informed, healthy, 22-year-old, 90 kg male student, who had never been diagnosed with parasitic infection. His participation was approved by the Human Experimentation Ethical Review Committee of the University of Queensland.
A total of 100 L3 in BU buffer (see Box 1) was swallowed in two gelatin capsules with food. From the third week after infection onwards, faecal microscopy for hookworm eggs was carried out at weekly intervals (see Box 1). Peripheral venous blood was collected on the day of infection and thereafter at either weekly or fortnightly intervals for 25 weeks. Routine white cell counts were performed.
In an attempt to minimise the confounding effects of persisting dormant larvae, at six months 200 mg albendazole was taken with a fatty meal twice daily for three days. While dietary lipid significantly increases the systemic availability of albendazole,6 the efficacy of this drug against dormant larvae remains uncertain.
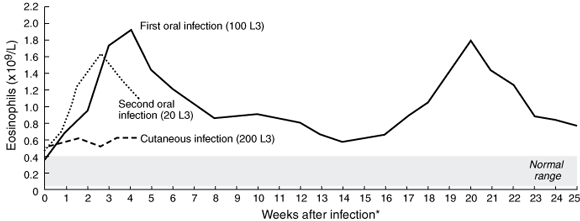
Experiment 1: After the first oral inoculation, a significant eosinophilic response was observed (Box 2), peaking at 1.91 × 109/L during Week 4 after infection and then gradually declining to a plateau at Week 9. Mild abdominal discomfort was experienced in the first three weeks of infection, but was never severe. There were no respiratory symptoms. At Week 14 (the beginning of spring), peripheral eosinophil levels started to climb rapidly, coupled with intermittent, mild abdominal pain. Eosinophilia peaked at 1.78 × 109/L by Week 20, then declined gradually to the earlier plateau levels (Box 2). The profile of the second eosinophil peak was almost identical to that of the first. Helminth eggs were not seen in faeces at any time.
Experiment 2: After cutaneous exposure, eosinophil levels rose only slightly (Box 2), although the infection site became painful and itchy for 10 days after infection, with gross erythema, vesiculation and exudation (but not frank suppuration), not suggestive of a secondary bacterial infection, but consistent with an intense, localised allergic response. Serpiginous cutaneous lesions did not develop.
Experiment 3: After the second oral dose (20 L3), blood eosinophilia followed a pattern almost identical to that in Experiment 1, peaking at 1.71 × 109/L in the third week after infection (Box 2).
Until now, it has simply been accepted that patients with eosinophilic enteritis caused by A. caninum acquired the infection percutaneously. However, there has still not been a documented case of human eosinophilic enteritis following intentional or known accidental percutaneous exposure to larvae of this parasite. Cutaneous exposure to large numbers of L3 in volunteers needed to be repeated many times to provoke a detectable serum antibody response, while blood eosinophilia appeared to require an even greater antigenic stimulus (P P, unpublished data).
By contrast, the oral route of infection used here, with small numbers of larvae, stimulated a dramatic peripheral eosinophil response. The peak eosinophilia of 1.91 × 109/L at Week 4 equals the highest levels reported from patients in Townsville and Brisbane diagnosed with eosinophilic enteritis.4 Given that the volunteer in this study had not been knowingly exposed to hookworms previously, such a level of eosinophilia is more likely to be a response to adult worms than to larvae. Certainly, L3 given by mouth to dogs will develop directly into adult worms,7 and the behaviour of A. caninum L3 in humans parallels that in adult dogs.2 Further, the time from infection to the first eosinophil peak, about four weeks, equals the presumed life expectancy of an adult A. caninum in human infections.2 While immunological monitoring would have been interesting, it was not available at the time. However, varying numbers of L3 may become dormant within the intestine or skeletal muscle to resume development in spring or after ingestion by a definitive host. The twin blood eosinophil peaks of Experiment 1 (Box 2) suggest the transient intestinal presence of an adult worm early in infection, with the subsequent mobilisation and development of dormant larvae leading to the second eosinophil peak. It would not be coincidental, then, that this synchronised with transient abdominal pain, possibly reflecting enteric inflammation. However, the symptoms were never severe enough to justify medical intervention (endoscopy or anthelmintic chemotherapy). That blood eosinophil levels did not return to normal between peaks, but remained at a high plateau, perhaps indicates that larvae in the gut or tissue reservoirs were stimulating an ongoing immune response.
Eosinophilic enteritis has relapsed in patients with previously documented A. caninum infection who had subsequently avoided re-exposure; mobilisation of dormant tissue larvae also probably explains the rising incidence of cases in early summer.8 Other causes of eosinophilia in our volunteer, such as allergy or drug reaction, are inconsistent with his medical history.
The lack of an eosinophil response to low-level percutaneous infection supports the conclusion that eosinophilia in Experiment 1 was triggered by adult rather than larval worms. Twice as many L3 were given in Experiment 2, producing intense cutaneous inflammation but no blood eosinophilia. The reaction following the second, smaller, oral dose of 20 L3 in Experiment 3 reinforces this supposition.
While our findings do not exclude percutaneous entry as a significant route of human infection leading to eosinophilic enteritis, they do indicate that ingestion of A. caninum larvae might be more pathogenic in that it leads to the direct development of adult worms in the gut, with its accompanying eosinophilic inflammation. Alternatively, tracheal migration following ingestion may occur, but, based on the low numbers of larvae required for oral infection and also the inability of much larger numbers of larvae applied percutaneously to complete this migration, we consider this less likely in this instance.
Possible routes of oral infection are by drinking soil-contaminated water, eating soil-contaminated food (eg, on fresh vegetables), or by eating infected meat. Many grazing animals and free-range poultry can become infected from soil contaminated by dogs. Ingestion of this meat, if undercooked, might lead to L3 developing directly into adult worms in the gut, as has been suggested for the very closely related, anthropophilic species Ancylostoma duodenale.9 Clearly, confirmation of this will require more extensive studies, including investigation of the possible role of paratenic hosts in human infection.
1: Details of isolation of third-stage larvae from infected dog faeces and of preparation of faecal samples from the human volunteer for microscopy
Isolation of larvae: Equal volumes of faeces and vermiculite were mixed well in a one litre plastic container, moistened and incubated at 30oC, being stirred daily. After five days, the mixture was spread onto large glass Petri dishes, covered with a 5 mm layer of washed, coarse river sand and overlaid with two layers of damp surgical gauze. The top layer of gauze (into which infective L3 had migrated) was removed (and replaced) at 12-hour intervals, to be rinsed in distilled water. Larvae were retrieved from the suspension by gravitational sedimentation, and stored in BU buffer (50 mM Na2HPO4, 22 mM KH2PO4, 70 mM NaCl) at 12oC to preserve their infectivity.5
Human faecal sample preparation: A 4 g sample of fresh faeces was comminuted in 4 mL ethyl acetate with 2 mL tap water, then centrifuged at 300 g for 10 minutes. The supernatant was discarded, while the sediment was resuspended in saturated MgSO4 solution (to fill the tube) and left to settle for 10 minutes. A coverslip was touched to the surface meniscus, then placed on a slide for examination by light microscopy.
Received 5 March 2002, accepted 12 September 2002
- Juergen K Landmann1
- Paul Prociv2
- Department of Microbiology and Parasitology, The University of Queensland, St Lucia, QLD.
We thank Debbie Laws and Rebekah Wilson from the School of Companion Animal Science, The University of Queensland, for providing access to infected dogs. Sullivan and Nicolaides Pathologists, Brisbane performed the routine white cell counts. Vicki Whitehall, the first author's wife (fiancée at the time), is thanked for allowing experimentation and sample collection in the bathroom of her rented townhouse.
None identified.
- 1. Prociv P. Zoonotic hookworm infections (Ancylostomosis) [Chapter 61]. In: Palmer SR, Lord Soulsby Simpson, DIH, editors. Zoonoses: biology, clinical practice, and public health control. Oxford: Oxford University Press, 1998: 803-822.
- 2. Prociv P, Croese J. Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a "new" zoonosis. Acta Trop 1996; 62: 23-44.
- 3. Schad GA, Chowdhury AB, Dean CG, et al. Arrested development in human hookworm infections: an adaptation to a seasonally unfavourable external environment. Science 1973; 180: 52-54.
- 4. Prociv P. Pathogenesis of human hookworm infection: insights from a "new" zoonosis. In: Freedman DO, editor. Chemical immunology: immunopathogenetic aspects of disease induced by helminth parasites. Basel: S Karger AG, 1997: 62-98.
- 5. Hawdon JM, Schad GA. Long-term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. J Helmin Soc Washington 1991; 58: 140-142.
- 6. Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol 1998; 34: 315-317.
- 7. Yokogawa S, Oiso T. Studies on oral infection with Ancylostoma. Am J Hyg 1926; 6: 484-497.
- 8. Croese J. Seasonal influence on human enteric infection by Ancylostoma caninum. Am J Trop Med Hyg 1995; 53: 158-161.
- 9. Schad GA, Murrell KD, Fayer R, et al. Paratenesis in Ancylostoma duodenale suggests possible meat-borne human infection. Trans R Soc Trop Med Hyg 1984; 78: 203-204.





Abstract
Objective: To investigate possible routes for human infection by the dog hookworm (Ancylostoma caninum).
Design, setting and participant: Relatively small numbers of infective larvae were administered orally and percutaneously to an informed healthy volunteer (J K L) under medical supervision, at intervals between May 1998 and May 1999.
Main outcome measures: Symptoms; weekly blood eosinophil counts; faecal microscopy.
Results: A marked blood eosinophilia followed a single oral exposure to 100 infective larvae, while faecal examination remained negative. Eosinophil counts then declined gradually, although a rapid, spontaneous rise several months later, at the beginning of spring, possibly indicated reactivation of dormant larvae. Blood eosinophil numbers did not rise significantly after percutaneous infection with 200 larvae. A subsequent, smaller, oral inoculum of 20 larvae provoked an eosinophil response similar to that of the first experiment.
Conclusions: Our findings suggest that, following ingestion, some infective larvae of A. caninum develop directly into adult worms in the human gut (as they do in dogs). While the percutaneous route might be the most common means of human exposure to canine hookworm larvae, leading generally to subclinical infection, oral infection may be more likely to provoke symptomatic eosinophilic enteritis.