Several studies have investigated whether restrictions on the availability of paracetamol in Europe and Australia have affected rates of mortality, hepatotoxicity and non-fatal self-poisoning from the drug.1-6 Restrictions in Europe have included a cap on the amount of paracetamol available at a single purchase without prescription, and limiting sales to blister packs.4 In France, paracetamol may only be purchased from pharmacies and the content of each pack is legally limited to eight grams.2
In Australia, following extortion threats to two pharmaceutical companies, products containing paracetamol were recalled on two occasions in 2000.6 We assessed the effect of the recall in Western Australia, where all admissions to hospital are recorded on a single database.
We extracted data from the Hospital Morbidity Data System, which records all admissions to private and public hospitals throughout WA. Diagnoses were coded using ICD-9-CM7 until 30 June 1999, and then ICD-10.8 We extracted all admissions for poisoning (ICD-9-CM, 960–979; ICD-10, T36–T50) from 1 January 1996 to 31 December 2001. Paracetamol overdoses were identified using the codes 965.4 (ICD-9-CM) and T39.1 (ICD-10). We also identified admissions where the principal poisoning agent was aspirin (ICD-9-CM, 965.1; ICD-10, T39.0) or ibuprofen (ICD-9-CM, 965.6; ICD-10, T39.3), as well as all other poisoning agents.
We considered intentional and unintentional overdoses in all age groups of all agents, including paracetamol and other over-the-counter analgesics, as it is difficult to distinguish between intentional and unintentional self-poisoning.9 In addition, the effect of paracetamol availability on accidental overdoses by children has only been considered in one previous study.6 We considered poisonings by all agents, as it has been suggested that restricting the availability of paracetamol may lead to an increase in deliberate self-harm involving other agents.6
Paracetamol manufactured by Herron (Tennyson, QLD) was recalled from 16 March to 21 May 2000, and paracetamol made by Smith-KlineBeecham (Boronia, VIC) from 6 June to 23 August 2000.6 The recall lasted 146 days, so each person in the population contributed 146 days at risk each year. The admission rate for overdoses was calculated during this period, and for the same period of other years. Admission rates were also calculated for the remainder of each year with the WA population (1.3 million) as denominator.10
As poisonings and attempts at deliberate self-harm may be subject to seasonal fluctuations, time series analysis techniques were used to test for any significant change in the rate of hospital admissions following overdose of paracetamol during the period of sales restriction. We used the X-11 procedure11 to identify any seasonal fluctuations and adjust the series accordingly. ARIMA modelling12 was used to test for any significant interruption to the time series during the period of restriction. All analyses were conducted using SAS software.13
There were 11 752 hospital admissions in WA for poisoning from 1996 to 2001. Paracetamol was the primary poisoning agent in 2266 (19.3%) admissions, aspirin in 120 (1%) and ibuprofen in 277 (2%). Admission rates for overdoses of each agent are shown in Box 1 in the recall period compared with the same period in each other year. There was a statistically significant decrease in the admission rate for paracetamol poisoning when sales were restricted in 2000 (Box 1). There was no increase in poisoning with other agents at this time.
These data were further analysed using weekly time series, as the recall period did not correspond with whole months. We found a non-significant reduction of 1.1 per week (95% CI, –2.4 to 0.1) in the admission rate for paracetamol poisoning, from an average of 7.3 per week (z = –1.69; P = 0.09). Paracetamol overdoses were no more or less common at any time of the year (Box 2). There were similar results when accidental poisonings and deliberate self-harm were considered separately. We found no increase in admission rates for poisoning with other agents while paracetamol was restricted (Box 3).
Although other studies have demonstrated an association between reductions in the availability of paracetamol and measures of poisoning,2-4 it is important to assess the effect on poisonings from other agents,5,6 and to assess random as well as seasonal variations in rates.
Our study had insufficient power to examine the effect on mortality rates. We were only able to measure the effect of the recall on poisonings requiring hospital admission and to establish a possible association rather than a causal relationship. Furthermore, we were unable to assess how sales of other paracetamol brands changed, and for the possibility that other factors influenced our results.
However, in contrast to a smaller study from New South Wales (n = 143),6 we found a significant fall in admissions for paracetamol overdose during the recall. This was not reflected in the same period in other years, thereby controlling for seasonal variation and reporting bias inherent in routine databases. Importantly, there was no increase in the admission rate for poisoning with other agents. However, admissions for paracetamol overdose showed a large random variation that tended to obscure any effect.
Our study suggests restrictions on paracetamol sales need to last for several months across all retail outlets for a more definite effect. Paracetamol availability was restricted in Australia for only four months, with the first recall affecting mainly supermarkets and the second pharmacies.6 Other studies that have found falls in measures of paracetamol poisoning have covered 4–36 months of restriction.2-5 The benefits of restriction may take several months to become apparent.
Variations in national rates of overdose may not be solely attributable to availability of paracetamol.9 Nevertheless, about half the people who overdose with paracetamol do not obtain it specifically to overdose, but use what is available.2 If packet sizes were limited, it is less likely that people would keep potentially lethal quantities at home.2 We believe that legislation to restrict availability of paracetamol should be considered given that voluntary codes of conduct have failed to reduce availability.14
1: Rate of hospital admission following overdoses, 1996–2001
|
Paracetamol available |
Paracetamol restricted (2000) |
|||||||||
Average/year |
Rate* |
Number |
Rate* |
Rate ratio |
(95% CI) |
P |
|||||
Aspirin |
7 |
0.96 |
7 |
0.93 |
0.97 |
(0.43–2.19) |
0.950 |
||||
Paracetamol |
151 |
20.56 |
127 |
16.90 |
0.82 |
(0.68–0.99) |
0.041 |
||||
Ibuprofen |
20 |
2.76 |
18 |
2.40 |
0.87 |
(0.53–1.43) |
0.581 |
||||
Other agent |
971 |
132.58 |
932 |
124.02 |
0.94 |
(0.87–1.00) |
0.062 |
||||
Sale of paracetamol was restricted between 16 March 2000 and 21 May 2000 and between 6 June 2000 and 23 August 2000. The same period in each other year was used for comparison. * Rate per 100 000 person-years at risk. |
|||||||||||
2: Hospital admissions following overdose of paracetamol: original and seasonally adjusted series
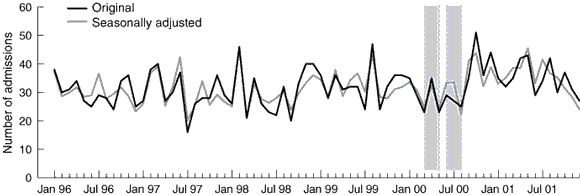
Sale of paracetamol was restricted between 16 March 2000 and 21 May 2000 and between 6 June 2000 and 23 August 2000 (shaded bars).
3: Weekly time series of hospital admissions following overdoses, 1996–2001
Type of poisoning |
Paracetamol available (1996–1999, 2001) (average admissions/week) |
Change when paracetamol restricted (2000) (average change/week) |
z |
P |
|||||||
Aspirin |
0.4 |
+ 0.02 |
+ 0.14 |
0.89 |
|||||||
Paracetamol |
7.3 |
– 1.10 |
– 1.69 |
0.09 |
|||||||
Ibuprofen |
0.99 |
– 0.12 |
– 0.46 |
0.65 |
|||||||
Other agent |
47.8 |
– 2.58 |
– 1.24 |
0.22 |
|||||||
Sale of paracetamol was restricted between 16 March 2000 and 21 May 2000 and again between 6 June 2000 and 23 August 2000. The same period in each other year was used for comparison. |
|||||||||||
- Stephen R Kisely1
- David Lawrence2
- Neil J Preston3
- 1 University Department of Psychiatry at Fremantle Hospital, University of Western Australia, Fremantle, WA.
- 2 Institute for Child Health Research, West Perth, WA.
- 3 Mental Health Directorate, Fremantle Hospital and Health Service, Fremantle, WA.
The Medical Research Foundation of Fremantle Hospital and the Department of Public Health, University of Western Australia, provided funding for the study.
None identified.
- 1. Fagan E, Wannan G. Reducing paracetamol overdoses. BMJ 1996; 313: 1417-1418.
- 2. Gunnell D, Hawton K, Murray V, et al. Use of paracetamol for suicide and non-fatal poisoning in the UK and France: are restrictions on availability justified? J Epidemiol Community Health 1997; 51: 175-179.
- 3. Prince MI, Thomas SH, James OF, Hudson M. Reduction in incidence of severe paracetamol poisoning. Lancet 2000; 355: 2047-2048.
- 4. Turvill JL, Burroughs AK, Moore KP. Change in occurrence of paracetamol overdose in UK after introduction of blister packs. Lancet 2000; 355: 2048-2049.
- 5. Hawton K, Townsend E, Deeks J, et al. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ 2001; 322: 1203-1207.
- 6. Balit C, Isbister G. Paracetamol recall: a natural experiment influencing analgesic poisoning. Med J Aust 2002; 176: 162-165.
- 7. National Coding Centre. The official NCC Australian version of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Sydney: National Coding Centre, 1996.
- 8. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD-10). Geneva: WHO, 1992.
- 9. Poulin C. Prevention of paracetamol poisoning. Lancet 2000; 355: 2009-2010.
- 10. Australian Bureau of Statistics. Population by age and sex, Western Australia. Perth: ABS, 1996–2001. (Catalogue no. 3235.5.)
- 11. US Bureau of the Census. The X-11 variant of the census method II seasonal adjustment program. Technical Paper No. 15, 1967 revision. Washington, DC: US Government Printing Office, 1967.
- 12. Box GEP, Jenkins GM, Reinsel GC. Time series analysis: forecasting and control. 3rd ed. Englewood Cliffs, NJ: Prentice Hall, 1994.
- 13. SAS System for Windows [computer program]. Version 8. Cary, NC, SAS Institute, 1999.
- 14. Slaven J, Kisely S. The Esperance primary prevention of suicide project. Aust N Z J Psychiatry 2002; 36: 617-621.





Abstract
Objectives: To assess the effect of two recalls of paracetamol products on rates of intentional and unintentional overdoses of paracetamol in all age groups, as well as any effect on poisoning by other agents.
Design: A before-and-after epidemiological study using data from the Western Australian Health Services Research Linked Database, which records all admissions to public and private hospitals throughout the State.
Main outcome measures: Hospital admissions in Western Australia for poisonings with all agents, including paracetamol and other over-the-counter analgesics.
Results: There were 11 752 admissions for poisoning from 1996 to 2001. Paracetamol was the primary poisoning agent in 2266 (19.3%) admissions, aspirin in 120 (1%) and ibuprofen in 277 (2%). There was a significant decrease in the admission rate for paracetamol poisoning when sales were restricted in 2000 (rate ratio, 0.82; 95% CI, 0.68–0.99) compared with the same period in other years. There was no increase in poisoning with other agents at this time. However, admissions for paracetamol overdose also showed a large random variation that tended to obscure any effect.
Conclusions: Our study highlights the need to control for random as well as seasonal fluctuations in admission rates, and for restrictions on paracetamol sales to last for several months across all retail outlets. Limiting access to paracetamol may reduce paracetamol poisonings without a coincident increase in the use of other agents.