Studies show that almost 50% of travellers returning to Europe and North America from the tropics experience health problems related to their travel, and that 8% consult doctors either abroad or on return.1 Figures are not available for Australian travellers but are probably similar. The most common diseases in travellers to the tropics are travellers' diarrhoea (35% incidence per month of travel), followed by acute respiratory infections (1.4%), giardiasis (0.7%) and other infections (including hepatitis, amoebiasis and gonorrhoea).1 Australian travellers increasingly visit tropical and developing countries and may present with infections that are rare in Australia.
Pre-travel health assessment and planning can prevent or ameliorate some of these problems (Box 1). Useful information about travel-related infections can be found at the websites of the World Health Organization (www.who.int/ith), United States Centers for Disease Control and Prevention (www.cdc.gov/travel) and the Department of Public Health and Tropical Medicine at James Cook University, Townsville (www.jcu.edu.au/school/phtm/PHTM/putravel.htm).
Over 90% of infections related to short-term travel present within six months of return. Diseases with long latent periods or potential for lifetime persistence are rare after short-term travel, but more common in those who have lived abroad or were born overseas. The usual presentation of a returned traveller is with a particular syndrome — fever, respiratory infection, diarrhoea, eosinophilia, or skin or soft tissue infection — or for screening for asymptomatic infection.
When assessing a returned traveller, it is important to obtain:
a complete travel history, including dates and places visited, and potential exposures to exotic diseases (eg, travel to rural areas, freshwater exposure, insect bites and sexual contacts); and
time of symptom onset in relation to travel, which may help eliminate diseases with different incubation periods (eg, fever beginning over 10 days after departure from an endemic area is unlikely to be dengue, which has a short incubation period). Incubation periods of common travel-related infections are shown in Box 2.
Between 2% and 12% of travellers suffer from a febrile illness while away or on return.1,3 Malaria is the most common diagnosis3-5 and is particularly frequent in travellers to West Africa who have taken no prophylaxis (2%).1 Among people arriving in Australia, the incidence of malaria is highest in those who have been in Nigeria (1.3%), the Solomon Islands (1.1%), Ghana (0.6%) and Papua New Guinea (0.4%).6 Other causes of fever are shown in Box 3. Younger travellers are more likely to have an exotic infection than the elderly, as their travel tends to be more adventurous. Older travellers are more likely to have a febrile complication of underlying disease, such as endocarditis or pneumonia.
Evaluation and initial management of fever in a returned traveller is outlined in Box 4. It is important to exclude falciparum malaria, which is potentially fatal but treatable. Once this is excluded, patients require hospital admission only if severely ill. Viral haemorrhagic fevers (eg, Lassa fever, and Marburg and Ebola virus infections) usually present as undifferentiated fever, but are rare in returned travellers. If suspected (recent travel to an African country with endemic disease or a known outbreak), then public health authorities must be notified to allow appropriate action.7
Malaria caused by Plasmodium falciparum generally presents within six weeks of return from an endemic area, although onset may be delayed by prophylactic mefloquine.8 In contrast, malaria caused by Plasmodium vivax may present many months after exposure. No signs or symptoms are specific to malaria, although fever is invariable. Occasionally, malaria mimics acute gastroenteritis, acute abdomen, pneumonia and meningoencephalitis. As prophylactic antimalarials do not eliminate the possibility of malaria, appropriate testing should always be done.
Malaria is diagnosed by detecting the parasite in a blood film or circulating malarial antigen. Initial blood films may be negative, and repeat films should be taken every six to 12 hours for 36–48 hours before malaria can be confidently excluded. As untreated falciparum malaria can be fatal within 24–48 hours of presentation, particularly in children, the diagnosis should be pursued urgently (case history, Box 5).
Patients with falciparum malaria require hospital admission until the disease is clearly under control. All should be treated as if they have chloroquine-resistant disease. Uncomplicated disease is treated with oral quinine plus either doxycycline or pyrimethamine–sulfadoxine, or, alternatively, mefloquine as a single agent, or the combination of atovaquone and proguanil.9 Severe malaria requires intravenous quinine.
Malaria caused by the Plasmodium species vivax, ovale and malariae can still usually be treated effectively with oral chloroquine followed by primaquine to eradicate the hepatic stages of P. vivax and P. ovale. Patients receiving primaquine should have prior testing for glucose-6-phosphate dehydrogenase deficiency, as the drug may cause severe haemolytic anaemia if this is present. However, as chloroquine-resistant P. vivax has now been reported throughout South-East Asia,10 specialist advice may be required, particularly for relapsing disease.9
Infected travellers have periodically introduced this common mosquito-borne flavivirus disease to northern Australia. Its range is currently spreading as one of its vectors, Aedes albopictus, becomes acclimatised to the urban environment.11
Clues to diagnosis of dengue are the short incubation period (four to seven days), maculopapular rash, thrombocytopenia and leukopenia. The last three features also occur in some rickettsial diseases (eg, scrub typhus), HIV seroconversion (Box 6), Q fever, meningococcal disease, toxic shock syndrome, measles, secondary syphilis and rubella. Diagnosis depends on virus isolation from blood, amplification of viral nucleic acid or serological results (dengue IgM or IgG seroconversion).
There is as yet no effective antiviral therapy, and supportive treatment is all that can be offered. Spontaneous resolution is usual. Occasionally, severe depression complicates the recovery phase.
Enteric fever is the clinical syndrome caused by Salmonella typhi or "paratyphi" Salmonella species. Fever is the dominant symptom, with gastrointestinal symptoms variably present. Typhoid vaccine confers about 70% protection against S. typhi, so does not rule out the diagnosis. Diagnosis depends on culture, as serological testing is unreliable, particularly if vaccine has been given.
In a patient with prolonged, undifferentiated fever, empirical therapy for enteric fever with quinolones (eg, ciprofloxacin) can be considered once malaria has been ruled out. Quinolone-resistant strains of Salmonella have now been reported, and, in children, third-generation cephalosporins (eg, ceftriaxone or cefotaxime) may be required.9
About 20%–40% of travellers have respiratory symptoms while away or on return.12,13 The most common causes are allergy and viral infection of the upper respiratory tract.
Tuberculosis (defined as skin test conversion) occurs in an estimated 2% of long-term travellers to high risk areas, such as sub-Saharan and North Africa, South and East Asia, the Middle East and Central and South America.14 Risk is lower during short-term holidays. Infants and children staying in these areas for more than three months are recommended to have bacille Calmette–Guérin (BCG) vaccination, as it reduces disease severity and mortality. For adults, the recommendations are less clear. Possible strategies include pre-travel BCG vaccination or Mantoux screening before and after travel, followed by prophylactic isoniazid for those whose skin test has converted. However, a recent analysis suggested that, given the problems of compliance and adverse effects with these strategies, it is more rational to await the rare cases of clinically overt travel-associated tuberculosis and treat appropriately as they arise.15
Acute diarrhoea is common in travellers to developing countries.16 Despite the advice "cook it, boil it, peel it or forget it", dietary transgressions are usually unavoidable. Most cases of travellers' diarrhoea are caused by bacterial contamination of food, particularly with enterotoxigenic Escherichia coli (ETEC). Other causes are shown in Box 7. It is rarely life-threatening. Mean duration of symptoms is four days. Up to 60% of people complain of cramps, 15% of bloody stools, and 10% of fever or vomiting.
Preventive strategies include education about safe food and beverages, water purification, and chemoprophylaxis with antibiotics (eg, trimethoprim–sulfamethoxazole or norfloxacin administered once daily). Antibiotics are 70%–95% effective in preventing travellers' diarrhoea,16 but should be considered only in selected high-risk, short-term travellers — those who cannot tolerate a brief illness (eg, athletes, business and political travellers) and those with increased susceptibility (eg, because of achlorhydria, gastrectomy, history of repeated severe travellers' diarrhoea, immunocompromise or chronic illness). Prophylaxis is not recommended for healthy travellers. As most travellers' diarrhoea occurs during travel, patients should be instructed in self-management (Box 8).
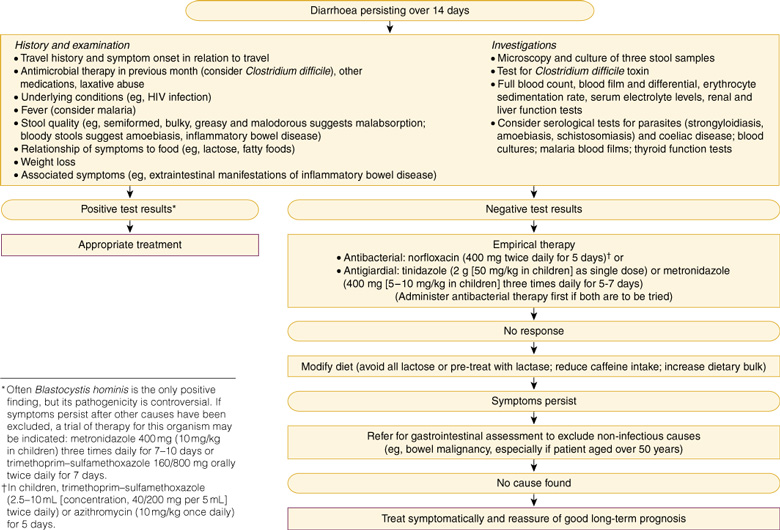
When diarrhoea persists for 14 days or more (1%–3% of cases18), bacterial causes are less likely. Parasite infestations are more common, but the spectrum of causes is much wider and includes non-infective causes, such as lactose intolerance, irritable-bowel syndrome, post-infectious malabsorption, bile-salt enteritis, inflammatory bowel disease, malignancy, coeliac disease and drugs. An approach to evaluation of persistent diarrhoea in travellers is shown in Box 9. It is important to consult with the pathology laboratory, as tests for some potential causes may not be routine.
Some patients have continuing symptoms (loose stools, urgency, and sensitivity to certain foods resulting in flatus or diarrhoea, similar to irritable-bowel syndrome) despite empirical therapy and negative results on gastroenterological workup. They are best treated symptomatically and reassured that the prognosis is good.18
Eosinophilia (blood eosinophil count ≥ 0.6 × 109/L) in a returned traveller may be an isolated finding or associated with symptoms such as cough, fatigue, skin lesions (urticaria), abdominal discomfort and diarrhoea. A recent German study of 14 000 travellers returning from the tropics found eosinophilia in almost 5%, and a definite diagnosis was made in a third of these.19 The higher the grade of eosinophilia, the more likely a travel-related diagnosis, particularly helminth infestation (eg, strongyloidiasis, filariasis, hookworm, schistosomiasis, cutaneous larva migrans and ascariasis).19 However, less than half of those with helminth infestation in the total population had blood eosinophilia. The absence of eosinophilia does not exclude parasites, as eosinophilia generally occurs only during the tissue invasive stages of worm development.
Initial investigation of eosinophilia should include three stool examinations for ova, cysts and parasites to detect the more common gastrointestinal helminths, whose eggs may be excreted intermittently. Patients may report passing worms in faeces or coughing them up, and any macroscopically visible worms (likely to be ascarids or tapeworm) should be sent for laboratory identification. Specific serological testing is available for schistosomiasis, strongyloidiasis, filariasis, echinococcosis, toxocariasis and angio-strongyliasis.
Initial empirical treatment with a broad-spectrum anthelmintic is reasonable after stool collection. Options include mebendazole, pyrantel and albendazole.9 As the first two are not effective for strongyloidiasis, tapeworm infection or schistosomiasis, a specific diagnosis should be attempted if the eosinophil count does not return to normal and treated appropriately. Albendazole is available from the Pharmaceutical Benefits Scheme on authority for specific indications.
Infection is acquired by penetration through intact skin of filariform larvae of Strongyloides stercoralis in faecally contaminated soil. Autoinfection may occur, and no intermediate host is necessary. Infection can persist for decades and occasionally presents as hyperinfection syndrome after immunosuppression.
Strongyloidiasis may be diagnosed with stool microscopy and special culture techniques, as well as serological testing. Effective treatment includes ivermectin, albendazole and thiabendazole.9 Repeat or prolonged treatment may be necessary in patients who are immunosuppressed.
Schistosomiasis detection is increasingly common in Australian travellers.20 Although the parasite is endemic in Africa, the Middle East, Latin America, and limited areas in Asia, the greatest risk of infection appears to come from exposure to freshwater in Africa, especially Lake Malawi. Infection results from penetration of intact skin by larvae liberated from snail intermediate hosts.
Most infected travellers are asymptomatic and at low risk of complications because of low parasite burdens, although end-organ damage may result from egg deposition. Other presentations include swimmers' itch (a papular pruritic dermatitis at the time of exposure) and Katayama fever, which is associated with the migration of schistosome larvae throughout the body (symptoms include fever, chills, malaise, headache, cough, abdominal pain, diarrhoea, urticaria, and occasionally neurological disorders).
Once egg deposition begins, clinical features include haematuria, haematospermia, urgency, frequency, terminal dysuria, salpingitis, prostatitis and genital ulcers (case history, Box 10). More serious but rare complications are paraparesis, paraplegia and mass cerebral lesions. Immigrants and refugees from endemic countries are likely to have much heavier infection loads and may present with the complications of chronic fibrosis, such as ureteric obstruction and portal hypertension.
The standard treatment is praziquantel (20 mg/kg orally for two doses, four hours apart). About 10%–15% of patients require re-treatment.
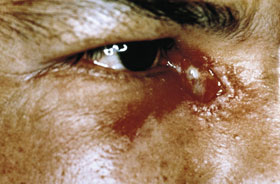
Although not usually serious or life-threatening, skin and soft tissue infections are common. Most common are the infections also seen in non-travellers, such as cellulitis, furunculosis, folliculitis and dermatophyte infections. Other infections are more exotic. For example, among 269 patients who presented to a Paris tropical disease unit with travel-associated dermatosis, 53% had an imported tropical disease.21 Most common among these were cutaneous larva migrans (25%; Box 11), pyodermas (18%), pruritic arthropod-reactive dermatitis (10%), myiasis (9%), tungiasis (6%), urticaria (5%), fever and rash (eg, dengue) (4%) and cutaneous leishmaniasis (3%; Box 12).
Non-healing skin lesions and furuncles in travellers from Africa and South America should be examined closely, as myiasis or infestation with fly larvae (eg, Cordylobia or African tumbu or putsi fly) is possible. The larvae are best removed by applying petroleum jelly in a toothpaste cap. They burrow into the cap and can then be extracted with forceps, the whole process usually taking less than an hour.
Sandfleas, or "jiggers", usually burrow into the skin of the soles of the feet and around toenails, causing itchy lumps. Prevention is by wearing shoes, avoiding sandals and bare feet. Jiggers are best shelled out, being careful not to break the developing egg sac of the female flea.
Returned travellers often present requesting a "check-up". Cost-effectiveness studies have yet to demonstrate the benefits of widespread screening for returned travellers.23
A careful history of potential exposures should include illnesses while away, sexual exposure, food, swimming in rivers and lakes, contact with animals, use of illicit drugs, compliance with antimalarial prophylaxis, and pre-travel vaccinations. Physical examination depends on history, and more directed tests for follow-up of disease experienced while away may be required.
Risk wanes with time for most infections but persists for life for some diseases, including:
Bacteria — Mycobacterium leprae, Mycobacterium tuberculosis, Treponema pallidum, Rickettsia prowazekii and Salmonella typhi;
Viruses — hepatitis B, C and delta viruses, herpes simplex viruses, HIV 1 and 2 and HTLV-1;
Protozoa — Plasmodium malariae, Toxoplasma gondii, Trypanosoma cruzi (Chagas' disease);
Helminths — Echinococcus granulosis, Strongyloides stercoralis, Taenia solium, Schistosoma spp.; and
Fungi: Coccidioides immitis, Histoplasma capsulatum.
Some of these infections may be latent and reactivated during immunosuppression.
Evidence-based recommendations
Fever in a traveller within three months of departure from a malaria-endemic area is a medical emergency and should be investigated with thick and thin blood films to exclude malaria; films should be repeated at least twice if negative and symptoms persist5 (E3).
The most important factors determining survival in falciparum malaria are early diagnosis and prompt initiation of appropriate treatment5 (E3).
Antimotility agents, such as loperamide, are safe and efficacious for mild to moderate cases of travellers' diarrhoea in adults16 (E1); most acute cases of this diarrhoea respond to a single dose or three-day course of antimicrobial therapy16 (E3).
Persistent diarrhoea in returned travellers is less likely to have an infective cause than acute diarrhoea and require investigation18 (E3).
Post-exposure rabies treatment is effective and should be considered for all travellers with potential exposure24 (E1).
1: Pre-travel health assessment and planning
This is recommended at least six weeks before departure and should include:
Assessment of fitness to travel: pre-existing conditions (eg, diabetes, heart disease, pregnancy, immunocompromise); psychological state (eg, depression, seeking "way-out" of problems, alcohol, drugs); need for specialised medications, with doctor's letter specifying this.
Full travel history and risk assessment
Update of routine vaccinations: measles–mumps–rubella; diphtheria; pertussis; tetanus; hepatitis B; poliomyelitis; influenza; and pneumococcal vaccines.
Routine vaccinations for travel to developing countries: hepatitis A and (if travelling for more than two weeks) typhoid.
"Special circumstance" vaccinations: yellow fever; meningococcus; rabies; and Japanese B encephalitis.
Prophylactic drugs when indicated: antimalarials (mefloquine, doxycycline, chloroquine, proguanil or malarone); and antibiotics for travellers' diarrhoea (if immunocompromised or other special circumstances).
Advice on prevention: travellers' diarrhoea; schistosomiasis and fresh-water exposure in Africa; traffic-accident injuries; sexually transmitted diseases; sunburn and sunstroke; insect bites; scuba-diving accidents; deep venous thrombosis; and jet lag.
Advice and drugs for medical or first-aid kit: including diarrhoea kit (loperamide, antibiotic [norfloxacin], thermometer and [for long-term traveller or trekker] tinidazole).
Advice on travel health insurance and assistance
2: Incubation periods of travel-related infections2
Short (< 10 days) |
Intermediate (10–21 days) |
Long (> 21 days) |
|||||||||
Influenza Dengue Yellow fever Plague Paratyphoid fever Mediterranean spotted fever African tick-bite fever Rocky Mountain spotted fever |
Malaria Viral haemorrhagic fevers Typhoid fever Scrub typhus Q fever Relapsing fever (Borrelia spp.) African trypanosomiasis |
Malaria Hepatitis A, B, C, E Rabies Schistosomiasis Leishmaniasis Amoebic liver abscess Tuberculosis Filariasis Brucellosis |
|||||||||
3: Causes of fever in Canadian and Australian studies of returned travellers
Percentage of fever cases |
|||||||||||
Diagnosis |
Canada |
Australia |
|||||||||
Undiagnosed |
25 |
9 |
|||||||||
Malaria |
32 |
27 |
|||||||||
11 |
18 |
||||||||||
Hepatitis |
6 |
3 |
|||||||||
Diarrhoeal illness |
5 |
14 |
|||||||||
Pyelonephritis |
4 |
1 |
|||||||||
Dengue |
2 |
8 |
|||||||||
Enteric fever |
2 |
3 |
|||||||||
Epstein–Barr virus infection |
2 |
0.4 |
|||||||||
Rickettsial infection |
1 |
2 |
|||||||||
Amoebiasis |
1 |
1 |
|||||||||
Tuberculosis |
1 |
0.4 |
|||||||||
Acute HIV infection |
0.3 |
0.4 |
|||||||||
Other infections |
4 |
10 |
|||||||||
Other causes |
4 |
3 |
|||||||||
* Including upper respiratory tract infection, bronchitis and pneumonia. |
|||||||||||
4: Evaluation and initial management of fever in a returned traveller*
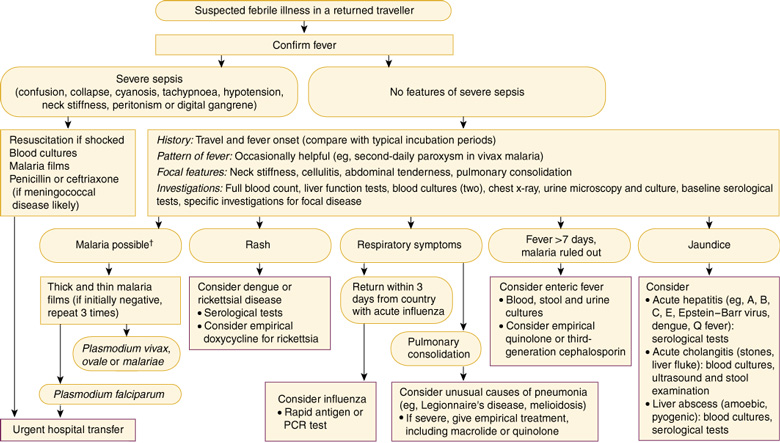
PCR = polymerase chain reaction. * Evaluation should also include the differential diagnoses that would be considered in a non-traveller with fever. † Travel to high-risk area, rural or prolonged travel, non-compliance with prophylaxis.
5: Case history — fever and confusion in a traveller to Papua New Guinea
History: A 42-year-old geologist presented 14 days after return from a 6-week field trip to Papua New Guinea. He had a six day history of high fevers and rigors. On the day of presentation, he had become vague and confused. He had taken antimalarials as prophylaxis, but ceased when he found that local people did not take them.
Examination: His temperature was 40oC, pulse rate 140 bpm, respiratory rate 28 per minute, and blood pressure 100/60 mmHg. He had dry mucous membranes, mild jaundice, pallor, splenomegaly and generalised crackles in both lungs. He was drowsy and disoriented but had no meningism.
Investigations: Full blood examination revealed reduced haemoglobin concentration (65 g/L; reference range [RR], 130–180 g/L), leukopenia (2.5 x 109/L; RR, 3.5–10 x 109/L) and thrombocytopenia (10 x 109/L; RR, 150–400 x 109/L). Malaria films were positive for Plasmodium falciparum, with 18% of red blood cells parasitised. Liver function tests revealed raised levels of bilirubin (60 μmol/L; RR, 3–20 μmol/L) and lactate dehydrogenase (489 U/L; RR, 100–225 U/L). Serum creatinine level was also raised (0.28 mmol/L [RR, 0.04–0.12 mmol/L]). Blood gas measurement revealed pH 7.1, Po2 110 mmHg (RR, 70–80 mmHg) and Pco2 15 mmHg (RR, 36–45 mmHg).
Management: Severe falciparum malaria was diagnosed. The patient was admitted to the intensive care unit, treated with ventilation, renal dialysis, intravenous quinine and exchange transfusion. Despite therapy and full support, he died on Day 5 of the admission.
Malaria should be excluded in any traveller with fever, even if prophylactic antimalarials have been taken, as their efficacy is less than 100%.
Severe malaria is defined by any degree of altered consciousness, focal neurological signs, jaundice, oliguria, severe anaemia, hypoglycaemia, hypotension, > 2% of red blood cells parasitised or absolute parasite count > 100 000/mL, vomiting or acidosis.
Severe malaria requires treatment with intravenous quinine and admission to an intensive care unit, as complications may occur suddenly, and quinine therapy must be monitored closely. Even with intensive-care treatment, mortality is high.
6: Case history — fever with rash after a tropical holiday
History: A 34-year-old man presented 10 days after returning from a six-week holiday in South-East Asia. He had an eight-day history of malaise, chills, headache, sore throat and generalised rash. He had stayed mostly at five-star hotels, but reported many mosquito bites.
Examination: He had fever, a macular rash and generalised lymphadenopathy with mild splenomegaly, but no meningism and no eschar present.
Investigations: Full blood examination revealed lymphocytosis with numerous atypical lymphocytes and thrombocytopenia. Blood cultures and malaria films were negative. Liver function tests revealed marginally elevated serum transaminase levels. Serological testing revealed past infection with Epstein–Barr virus and cytomegalovirus and was negative for Q fever, dengue, rubella, measles and rickettsial infection.
Management: The presumptive diagnosis was dengue, and supportive treatment was instituted. However, symptoms persisted for the next three weeks.
Dengue serological testing was repeated and found to be still negative.
A repeat sexual history revealed unprotected intercourse with a number of men during the holiday. HIV antibody tests were positive on enzyme immunoassay and indeterminate on western blot. A p24 antigen test was positive. HIV seroconversion illness was diagnosed, and appropriate counselling and therapy offered.
Acute HIV infection may mimic several tropical diseases, including dengue and typhus, as well as infectious mononucleosis.
A sexual history is important in assessing infection risk in any traveller with a febrile illness.
Early diagnosis of HIV infection is important in preventing transmission, as well as allowing the correct timing of antiretroviral therapy.
7: Causes of travellers' diarrhoea (% of cases)16
Bacteria (> 80%) Salmonella spp. Campylobacter spp. Shigella spp. Aeromonas spp. Plesiomonas spp. Yersinia spp. Vibrio cholerae Vibrio parahaemolyticus and other non-cholera vibrios Escherichia coli (eg, enterotoxigenic strains) Clostridium perfringens Clostridium difficile Edwardsiella tarda |
Viruses (0–36%) Rotavirus Adenovirus Caliciviruses Astroviruses Norwalk virus Parasites (0–20%) Giardia lamblia Cryptosporidium parvum Cyclospora cayetanensis Entamoeba histolytica Isospora belli Microsporidia Blastocystis hominis (pathogenicity controversial)17 |
8: Self-management of acute travellers' diarrhoea
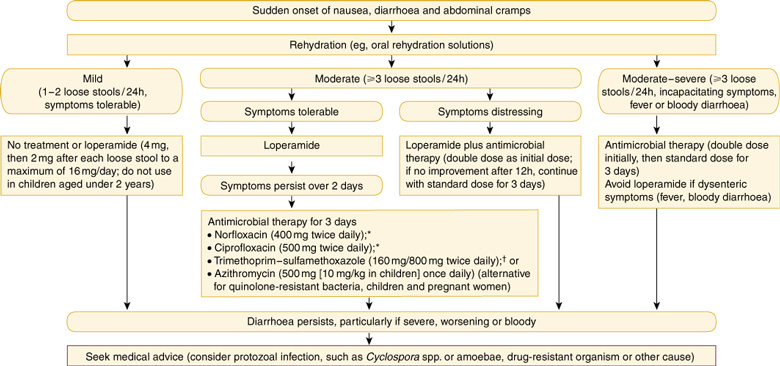
* Contraindicated in children under 16 years and pregnant women. However, for children with severe diarrhoea, the benefits of quinolones used for 1–3 days may outweigh the risks. † Although resistance to trimethoprim–sulfamethoxazole is widespread in some areas, prescription is less restricted for these antibiotics than for quinolones.
10: Case history — genital ulceration two years after a trip across Africa
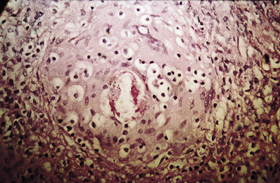
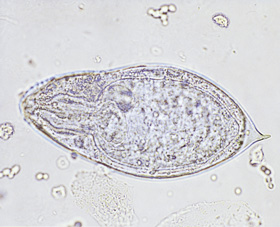
Presentation: A 28-year-old woman presented with a non-healing genital ulcer. She had travelled overland through Africa two years previously. Examination: An indurated 1 cm ulcer was present on the inner aspect of the left labia majoris. Investigations: Polymerase chain reaction tests were negative for herpes simplex, gonorrhoea and chlamydia; syphilis serological testing and a swab for Haemophilus ducreyi (chancroid) were also negative. Skin biopsy of the lesion revealed a granuloma surrounding a schistosome egg (pictured). Schistosoma haematobium eggs were detected in terminal urine collected between midday and 2 pm (pictured). Eosinophil count in blood was 1.2 x 109/L (reference range, < 0.6 x 109/L). Serological testing by indirect hemagglutination assay was positive for schistosomiasis (titre, 512). Management: She was treated with praziquantel and six months later remained well. Her asymptomatic travelling companions were found to be positive for schistosomiasis on serological screening and were also treated.
|
11: Cutaneous larva migrans in a traveller to Malaysia
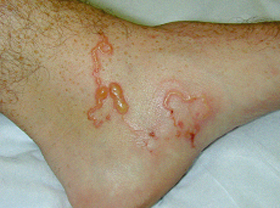
Cutaneous larva migrans is caused by the penetration through intact skin of larval animal hookworms. Diagnosis is predominantly clinical. Treatment is often necessary because of intense pruritus, long duration (over a year) and complications, such as impetigo and allergic reactions. Therapy comprises ivermectin, albendazole or thiabendazole.22
- David F M Looke1
- Jennifer M B Robson2
- Series Editors:
- 1 Infection Management Services – Southern Queensland, Princess Alexandra Hospital, Woolloongabba, QLD.
- 2 Sullivan Nicolaides Pathology, Taringa, QLD.
- 1. Steffen R, DuPont HL. Manual of travel medicine and health. Hamilton, BC: Decker, 1999.
- 2. Yung AP, Ruff T. Infections in returned travellers and immigrants. In: Yung AP, McDonald MI, Spelman DW, et al. Infectious diseases: a clinical approach. Melbourne: Royal Melbourne Hospital, 2001: 366-377.
- 3. Doherty JF, Grant AD, Bryceson ADM. Fever as the presenting complaint of travellers returning from the tropics. Q J M 1995; 88: 277-281.
- 4. MacLean JD, Lalonde RG, Ward B. Fever from the tropics. Travel Med Advisor 1994; May: 27.1-27.14.
- 5. O'Brien D, Tobin S, Brown G, Torresi J. Fever in returned travelers: review of hospital admissions for a 3-year period. Clin Infect Dis 2001; 33: 603-609.
- 6. Bryan J, Fa'afoi E, Forsyth S. Report of the Australian Malaria Register for 1992 and 1993. Commun Dis Intell 1998; 22: 237-245.
- 7. Isaacson M. Viral hemorrhagic fever hazards for travelers in Africa. Clin Infect Dis 2001; 33: 1707-1712.
- 8. Reyburn H, Behrens RH, Warhurst D, Bradley D. The effect of chemoprophylaxis on the timing of onset of Falciparum malaria. Trop Med Int Health 1998; 3: 281-285.
- 9. Therapeutic Guidelines Writing Group. Therapeutic guidelines: antibiotic. 11th ed. Melbourne: Therapeutic Guidelines Limited, 2000.
- 10. Barat LM, Bloland PB. Drug resistance among malaria and other parasites. Infect Dis Clin North Am 1997; 11: 969-987.
- 11. Jelinek T. Dengue fever in international travelers. Clin Infect Dis 2000; 31: 144-147.
- 12. Looke DFM, Mills DJ, Kass RB, et al. The "welcome home" letter and questionnaire as a valuable quality assurance tool for an Australian traveller's medical clinic. In: Lobel HO, Steffen R, Kozarsky PE. Travel medicine 2. Proceedings of the Second Conference On International Travel Medicine; Atlanta, Ga USA, 1991 May 9-12. Atlanta: International Society of Travel Medicine, 1991: 287-289.
- 13. Hill DR. Health problems in a large cohort of Americans travelling to developing countries. J Trav Med 2000; 7: 259-266.
- 14. Cobelens FGJ, van Deutekom H, Draayer-Jansen IWE, et al. Risk of infection with Mycobacterium tuberculosis in travellers to areas of high endemicity. Lancet 2000; 356: 461-465.
- 15. Rieder HA. Risk of travel-associated tuberculosis. Clin Infect Dis 2001; 33: 1393-1396.
- 16. Ericsson CD, DuPont HL. Travelers' diarrhea: approaches to prevention and treatment. Clin Infect Dis 1993; 16: 616-624.
- 17. Shlim DR, Hoge CW, Rajah R, et al. Is Blastocystis hominis a cause of diarrhea in travellers? A prospective controlled study in Nepal. Clin Infect Dis 1995; 21: 97-101.
- 18. DuPont HL, Capsuto EG. Persistent diarrhea in travellers. Clin Infect Dis 1996; 22: 124-128.
- 19. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis 2002; 34: 407-411.
- 20. Robson JMB, Htut T. Schistosomiasis — a need for increasing vigilance. Commun Dis Intell 1994; 18: 652-654.
- 21. Caumes E, Carriere J, Guermonprez G, et al. Dermatoses associated with travel to tropical countries. A prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin Infect Dis 1995; 20: 542-548.
- 22. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis 2000; 30: 811-814.
- 23. MacLean JD, Libman M. Screening returned travellers. Infect Dis Clin North Am 1998; 12: 431-433.
- 24. World Health Organization. Recommendations on rabies postexposure treatment and the correct technique of intradermal immunization against rabies. Geneva: WHO, 1996.





Abstract
The usual presentation of a returned traveller is with a particular syndrome — fever, respiratory infection, diarrhoea, eosinophilia, or skin or soft tissue infection — or for screening for asymptomatic infection.
Fever in a returned traveller requires prompt investigation to prevent deaths from malaria; diagnosis of malaria may require up to three blood films over 36–48 hours.
Diarrhoea is the most common health problem in travellers and is caused predominantly by bacteria; persistent diarrhoea is less likely to have an infectious cause, but its prognosis is usually good.
While most travel-related infections present within six months of return, some important chronic infections may present months or years later (eg, strongyloidiasis, schistosomiasis).
Travellers who have been bitten by an animal require evaluation for rabies prophylaxis.