Indigenous Health Research
Reducing premature death and renal failure in Australian Aboriginals
A community-based cardiovascular and renal protective program
Wendy E Hoy, Philip R Baker, Angela M Kelly and Zhiqiang Wang
MJA 2000; 172: 473-478
Abstract - Methods - Results - Discussion - Acknowledgements - References - Authors' details
- - More articles on Aboriginal health
Abstract Objective: To describe results of a systematic treatment program to modify renal and cardiovascular disease in an Aboriginal community whose rates of renal failure and cardiovascular deaths are among the highest in Australia.
Design: Longitudinal survey of people during treatment, and comparison of rates of natural death and renal failure with those in a historical control group.
Setting: Tiwi Islands (population, about 1800), November 1995 to December 1998.
Participants: All adults with blood pressure ≥ 140/90, with diabetes and urinary albumin/creatinine ratio (ACR) ≥ 3.4 g/mol (microalbuminuria threshold), or with progressive overt albuminuria (ACR ≥ 34 g/mol) were eligible for treatment. The historical control group comprised 229 people who satisfied these criteria in the pretreatment period 1992-1995.
Interventions: Perindopril, combined with calcium-channel blockers and diuretics if needed to achieve blood pressure goals; attempts to improve control of blood glucose and lipid levels; health education.
Main outcome measures: Blood pressure, ACR, serum creatinine level and glomerular filtration rate (GFR) over two years of treatment; rates of renal failure and natural death compared with control group (analysed on intention-to-treat basis).
Results: 258 people enrolled in the program, and 118 had complete data for two years of treatment. In these 118, blood pressures fell significantly, while ACR and GFR stabilised. Rates of the combined endpoints of renal failure and natural death per 100 person-years were 2.9 for the treatment group (95% CI, 1.7-4.6) and 4.8 for the control group (95% CI, 3.3-7.0). After adjustment for baseline ACR category, the relative risk of the treatment group versus the control group for these combined endpoints was 0.47 (95% CI, 0.25-0.86; P = 0.013). Treatment benefit was especially marked in people with overt albuminuria or hypertension and in non-diabetic people. The estimates of benefit were supported by a fall in community rates of death and renal failure.
Conclusions: Aboriginal people can participate enthusiastically in chronic disease management, with rapid, dramatic improvement in clinical profiles and mortality. Similar programs should be introduced urgently into other Aboriginal communities nationwide.
Aboriginal people in the Northern Territory are experiencing an epidemic of cardiovascular disease (CVD) and end-stage renal disease (ESRD). Age-standardised CVD death rates are three times those of non-Aboriginal people,1 while the incidence of treated ESRD in Aboriginal people is approaching 1000 per million, and doubling every four years.2 ESRD treatment costs, at $100 000 per person annually, are becoming a huge burden,3 but premature death is the greater human catastrophe.
These problems are especially serious in the communities of the Tiwi Islands, north of Darwin (population, about 1800) (Box 1). The incidence of ESRD among Tiwi people recently reached 2700 per million, and they have one of the highest CVD mortality rates in Australia.2,4In a community-wide screening program starting in the early 1990s, we found a high prevalence of cardiovascular risk factors, including type 2 diabetes and hypertension, and albuminuria (measured by the albumin/creatinine ratio (ACR) of a random urine specimen).5 Albuminuria correlated inversely with glomerular filtration rate (GFR), and its intensity predicted not only renal failure, but also cardiovascular deaths and all-cause natural deaths.6-9
In the early 1990s, use of antihypertensive drugs was increasing gradually in the Tiwi communities, but systematic management of the huge burden of morbidity identified by the screening program was beyond the capacity of the existing health services. In November 1995, we therefore introduced a systematic treatment program to reduce blood pressure and to modify the expression and progression of renal and cardiovascular disease. We describe the results of this program to the end of 1998.
Methods The study was a longitudinal survey of people in the Tiwi Islands communities during treatment, and comparison of endpoints with a historical control group. Treatment was offered to eligible people as part of improved standard care. All participants gave informed consent to have their course followed up for the projects The epidemiology and prevention of Aboriginal renal disease, Parts 1 and 2. These projects were approved by the Joint Institutional Ethics Committee of the Menzies School of Health Research and Territory Health Services, Darwin, and its Aboriginal subcommittee, and by the Tiwi Land Council (Part 1) and the Tiwi Health Board (Part 2).
Treatment program The program relied considerably on screening and treatment algorithms. Interventions included education about diet, exercise, health behaviours and medical treatment. Medical treatment centred around use of a long-acting angiotensin-converting enzyme inhibitor (ACEi) (perindopril; Coversyl [Servier]), aggressive blood pressure control,10,11 and, where appropriate, oral hypoglycaemic and lipid-lowering drugs. The choice of an ACEi was based on the well recognised antihypertensive and cardiovascular-protective effects of this class of drug12 and several reports, subsequently substantiated, of an additional renal protective effect.13-23
If antihypertensive drugs had been prescribed before entry into the study, they were discontinued or tapered when perindopril was started. Objectives were to achieve a minimum daily dose of 4 mg perindopril and to lower blood pressure, initially to < 130/85, but more recently to < 120/75.10 A stepped approach to achieve these blood pressures included increasing perindopril to 8 mg, with addition of long-acting calcium-channel blockers and/or diuretics if needed.
Participants were seen at least monthly while medications were introduced or changed, then at least every three months for the first year, and at least every six months thereafter. Each examination included a minimum of a brief history, medication review, and measurement of weight, blood pressure, urinary ACR and serum creatinine level and, in diabetics, evaluation of blood glucose control.
After a start-up period, the day-to-day program was largely conducted by local health workers and community project officers, who were supported by telephone contacts and regular visits by nurse coordinators from Darwin. Doctors, who reviewed eligibility assessments, supported or made treatment decisions and modified the protocols, were less intensively involved. The program has run in parallel with other clinic activities in Nguiu, Bathurst Island, but has been integrated into regular clinic activities at the Melville Island communities of Milikapiti and Pirlangimpi.
Participants
Treatment group: People eligible for ACEi therapy were those with:
- hypertension (blood pressure ≥ 140/90 mmHg);
- diabetes and ACR ≥ 3.4 g/mol (microalbuminuria threshold), regardless of blood pressure; or
- progressive overt albuminuria (ACR ≥ 34 g/mol on first testing and increasing over time), regardless of blood pressure or diabetes status.
All qualifying features needed to be confirmed on at least two occasions.
People with past adverse reactions and breastfeeding women were ineligible for ACEi therapy. Fertile women were advised about teratogenic risks and the options of contraception or discontinuation of ACEi medication early in unplanned pregnancy. People with serum creatinine levels over 250 µmol/L were considered ineligible for long-acting ACEi therapy in the first six months of the program, but were later enrolled when treatment proved safe and effective in people with mild and moderate renal insufficiency.
To some extent, enrolment was prioritised by disease severity. Thus, most people with overt albuminuria, uncontrolled blood pressure and renal insufficiency were enrolled in the first year of the program.
Control group: In the absence of a parallel control group, rates of renal failure and natural death in participants were compared with those of a historical control group from the pre-program period. This control group comprised adults whose results on a single screening examination between July 1992 and September 1995 met the eligibility criteria later used for the treatment program. Selection was blinded to their future course, which was followed to 30 October 1995.
Data analyses Analyses were performed using STATA statistical software.24 Clinical profiles in the treatment group were described at baseline, six, 12, and 24 months of treatment, regardless of compliance, and were compared by analysis of variance, using geometric means for ACR and serum creatinine level to normalise their distribution. All endpoint data in the treatment group were analysed on an intention-to-treat basis. Rates of natural death and renal failure were calculated by baseline ACR category for the intention-to-treat and control groups, and the risk ratios for the intention-to-treat group calculated in stratified analysis by ACR category by the Mantel-Haenszel method for cohort studies. Kaplan-Meier survival curves for both groups were derived, and survivals compared by the non-parametric Wilcoxon technique.
Results Enrolment By 31 December 1998, 258 people had enrolled in the program (29% of all adults in the island communities) and 227 were still participating. Of these, 39 had completed over three years of treatment, 137 over two years, 168 over one year, and 192 over six months. Of 31 dropouts, nine had died, seven had begun dialysis (two of whom later died), seven had stopped taking the medication because of side effects (cough in four; angioedema, itching and dizziness in one each), four became normotensive without treatment, two chose to quit, one moved, and one entered palliative care with osteomyelitis of the skull.
Characteristics of people who enrolled are shown in Box 2: 42% had diabetes, almost two-thirds had hypertension, with a quarter already prescribed enalapril, and almost two-thirds had overt albuminuria.
Medications and participation Doses of perindopril prescribed for the 227 people participating at the end of 1998 were 2 mg (5 people; 2%), 4 mg (72; 32%), and 8 mg (150; 66%). Calcium-channel blockers were being taken by 37 people (16%), diuretics by 15 (7%), and both by 13 (6%). Participation was enthusiastic, and compliance increased over time; 65% were taking ≥ 70% of their prescribed dose (assessed by pill counts and interview), 27% were taking medicine occasionally, and 7% were taking little or no medication at the end of 1998.
Two-year clinical profiles Of the 137 people who had been treated for at least two years, 118 had largely complete follow-up data and were included in the two-year profile. These 118 were well matched with participants not included in this profile for age, BMI, and blood pressure, but were more likely to have diabetes, overt albuminuria, and to have been taking prior ACEi therapy (Box 2). These differences reflected prioritisation of sicker people for early entry into the program.
Two-year clinical profiles for the 118 people are shown in Box 3. Treatment was associated with a swift and sustained fall in blood pressure, as well as stabilisation of ACR and GFR. Results are presented according to participants' clinical categories at baseline in Box 4. The fall in blood pressure was marked in people with hypertension at baseline and less marked but still apparent in those who had been normotensive, as well as in those previously prescribed an ACEi. Good blood pressure responses were seen in people both with and without diabetes, those with micro- and overt albuminuria and those with "normal" and raised levels of serum creatinine. Stabilisation of ACR and GFR was seen in all clinical categories. Indeed, serum creatinine level tended to fall and GFR to rise in all categories.
Baseline weight did not change (mean, 74 kg; SD, 16 kg), while mean serum potassium level rose non-significantly from 4.04 mmol/L (SD, 0.46 mmol/L) to 4.14 mmol/L (SD, 0.49 mmol/L). No one developed significant hyperkalaemia. There was no evidence that ACEi therapy accelerated progression to renal insufficiency.
Comparisons with control group Two hundred and twenty-nine people qualified as controls from the pre-program period, comprising 123 people who subsequently went onto the treatment program and 106 people who did not. Reasons for not going onto the program included death, dialysis, failure to qualify on subsequent examinations, presence of exclusion criteria (eg, pregnancy, breastfeeding), declining treatment, or moving.
Baseline characteristics of the control and intention-to-treat groups are compared in Box 2. The control group was younger at enrolment, had lower BMI, and included fewer people with diabetes or overt albuminuria. The control group was followed up for a total of 564 years (individual mean, 2.5 years; range, 1 month to 3.3 years) and the intention-to-treat group for 560 years (individual mean, 2.2 years; range, 2 weeks to 3.1 years).
Endpoints of the two groups are compared in Box 5. The treatment group as a whole had lower rates of dialysis, natural death and the combined endpoint (dialysis or death) than the control group, although the differences were not significant. However, rates of endpoints were strongly correlated with baseline ACR category. Indeed, renal failure necessitating dialysis was confined to people with ACR ≥ 100 g/mol at baseline, and in these people the treatment group had an estimated 57% lower dialysis rate than the control group. In contrast, rates of natural death and of the combined endpoint were lower in the treatment group than in the control group for all categories of baseline overt albuminuria. After adjustment for ACR category, the treatment group had an estimated 45% lower rate of natural death and an estimated 53% lower rate of the combined endpoint.
Box 6 shows estimates of the survival advantage in people with various baseline clinical profiles after adjustment for ACR category. Treatment benefit was strong in people with overt albuminuria, non-diabetic people and people with hypertension. It was less marked in diabetic or normotensive people.
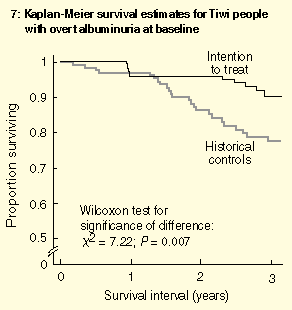
Survival estimates for people with overt albuminuria at baseline are shown in Box 7. Although the intention-to-treat group showed attrition during the first year (representing ESRD and deaths of seriously ill people prioritised for early entry), a survival advantage over the control group was clear by two years of the treatment program.
Discussion This study found that the introduction of a systematic treatment program to the Tiwi Island communities was associated with marked improvements in blood pressure and stabilisation of renal function in people receiving treatment. These changes contrasted sharply with the increase in blood pressure and ACR and fall in GFR noted previously in people matched for ACR category in the pretreatment status quo.10
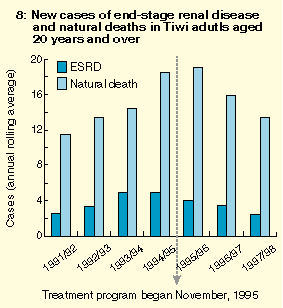
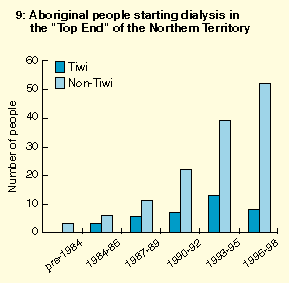
The treatment program was also associated with a swift and dramatic decrease in rates of renal failure and natural death in the treated group compared with a historical control group, suggesting that the program prevented or at least delayed these outcomes. Further evidence for the existence of this estimated survival benefit was the decrease in community-wide rates of ESRD and natural death -- previously increasing -- after introduction of the program (Box 8). In contrast, ESRD continued to increase among non-Tiwi Aboriginal people in the Top End (Box 9), arguing against a chance background effect. Preliminary estimates of cost effectiveness of the program, based solely on avoidance or delay of dialysis, are already startling.3,25
These results show that Aboriginal people are interested in health issues and receptive to health messages, and will take medications over the long term to protect against future health risk, with excellent response.
They also show that a systematic approach, with testing and treatment algorithms and clear goals, is superior to the previous approach of gradually improving medical management. While we cannot apportion relative benefit to individual elements of the treatment program, the observed fall in blood pressures alone would be expected to markedly reduce cardiovascular deaths and progression of renal disease,10,11 compatible with the effects we found.
Our intention-to-treat analyses probably underestimate the therapeutic efficacy of treatment, as a third of the intention-to-treat group took the prescribed medications only occasionally or not at all. Use of the historical control group was also a potential source of bias. On the one hand, it may have also led to underestimates of treatment benefit because of the group's potentially better survival prospects, based on its younger mean age, milder disease and the probable inclusion of people with borderline blood pressure or ACR readings, as eligibility for the group was not confirmed by a second examination. On the other hand, the 123 controls who subsequently entered the treatment program might have had superior survival characteristics to the controls who did not enter the program, potentially inflating the apparent benefit of the program.
Another source of bias was the prioritisation of the sickest people for early enrolment in the treatment program, many of whom were failing previous management regimens. This predisposes to poor short term outcomes of the program and underestimates of its benefits. Analyses of program results at four and five years, when more people have passed through one to two years of treatment, will dilute the impact of these early events. Longer-term analyses will also be needed to evaluate any survival effect of treatment in people without overt albuminuria, and the extent to which treatment has delayed rather than prevented ESRD and death in people with overt albuminuria.
The program could still be improved. Blood pressure control should be better; at two-year follow-up, 31% of people had blood pressures ≥ 140/90, and 50% had blood pressures ≥ 120/75.10 Hypertension, and therefore eligibility for treatment even in the absence of albuminuria, should probably be redefined as blood pressures ≥ 130/80 in this high-risk population.10 Control of blood glucose and lipid levels needs to improve. Finally, we might reassess the notions of the maximally renal-protective dose of ACEi and/or add other renal-protective drugs, such as angiotensin II receptor blocking agents,26,27 for poor responders.
Much of the success of this particular program derives from a strong sense of community ownership and control, a non-judgemental, non-authoritarian style, and respect for competing personal and community perspectives and priorities. Individuals appreciate personalisation of their health goals, and many are slowly adopting lifestyle changes.
This program is now being integrated into normal clinic activities at Nguiu. Its protocols have also been incorporated into standard care guidelines for Aboriginal adults in the Top End of the NT.28 Extension of its principles to other Aboriginal communities with high burdens of disease nationwide is a matter of urgency.29 Allocation of adequate resources is a challenge, but the clinical benefit and cost-effectiveness mandate the short- and intermediate-term investment.
Acknowledgements This study was supported by Servier Australia, the Australian Kidney Foundation, Rio Tinto, the National Health and Medical Research Council, the Stanley Tipiloura Fund, and Territory Health Services.
We gratefully acknowledge the support, enthusiasm and participation of the Tiwi community and the staff of the Tiwi Island clinics at Nguiu, Milikapiti and Pirlangimpi. We especially thank the Tiwi Health Board for review of this manuscript, and Treatment Program Coordinators Susan Jacups and Kiernan McKendry, Aboriginal Health Workers Jerome Kerinauia and Nellie Punguatji, and Community Project Officers Eric Tipiloura and Elizabeth Tipiloura for their dedicated work. Finally, we thank Resident Medical Officer, Dr Chris Harrison, for his support and participation. Dr Alan Cass updated the Top End ESRD rates.
- Cunningham J, Condon J. Premature mortality in Aboriginal adults in the Northern Territory. Med J Aust 1996; 165: 309-312.
- Spencer JS, Silva D, Hoy WE. An epidemic of renal failure among Australian Aborigines. Med J Aust 1998; 168: 537-541.
- You J, Hoy W, Beaver C, Zhao Y. Costs of hemodialysis and hospitalisations for patients with end stage renal disease in the Top End of the Northern Territory. Presented at the 35th Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology; 1999 Mar 3-5; Brisbane (Qld).
- Jain SK, editor. Trends in mortality by causes of death in Australia, the States and Territories during 1971-1992, and in statistical subdivisions during 1991-1992. Canberra: National Center for Epidemiology and Population Health and Australian Bureau of Statistics, 1994. (ABS catalogue no. 3313.0)
- Hoy WE, Pugsley DJ, Normal RJ, Hayhurst BG. A brief heath profile of adults in a Northern Territory Aboriginal community: with an emphasis on preventable morbidities. Aust N Z J Public Health 1997; 21: 121-126.
- Hoy WE, Mathews JD, Pugsley DJ, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in a high risk Aboriginal community. Kidney Int 1998; 54: 1296-1304.
- Cockcroft D, Gault MK. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41.
- Hoy WE, Wang Z, Baker P, et al. The natural history of renal disease in an Australian Aboriginal community. Presented at the 35th Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology; 1999 Mar 3-5; Brisbane (Qld).
- McDonald S, Wang Z, Hoy WE. Physical and biochemical predictors of death in an Australian Aboriginal cohort. Clin Exp Pharmacol Physiol 1999; 26: 618-621.
- The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VI). Arch Intern Med 1997; 157: 2413-2446.
- Collins R, Peto R, MacMahon S. Blood pressure, stroke and coronary artery disease. Part 2. Short term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990; 335: 827-838.
- Lonn EM, Yusuf S, Jha P. Emerging role of angiotensin converting enzyme inhibitors in cardiac and vascular protection. Circulation 1994; 90: 2056-2068.
- Mogensen CE. Angiotensin converting enzyme inhibitors and diabetic nephropathy. BMJ 1992; 304: 327-328.
- Ravid M, Savin H, Lang R, et al. Proteinuria, renal impairment, metabolic control, and blood pressure in type 2 diabetes mellitus. A 14-year follow up report on 195 patients. Arch Intern Med 1992; 152: 1225-1229.
- Ravid M, Savin H, Jutrin I, et al. Long term stabilizing effect of angiotensin converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type 2 diabetic patients. Ann Intern Med 1993; 118: 577-581.
- Gansevoort RT, de Zeeuw D, de Jong PE. Long term benefits of the antiproteinuric effect of angiotensin converting enzyme inhibition in nondiabetic renal disease. Am J Kidney Dis 1993; 22: 202-206.
- Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin converting enzyme inhibition on diabetic nephropathy. N Engl J Med 1993; 329: 1456-1462.
- Bedogna V, Valvo E, Casagrande P, et al. Effect of ACE inhibition in normotensive patients with chronic glomerular disease and normal renal function. Kidney Int 1994; 38: 101-107.
- Cattran DC, Greenwood C, Ritchie S. Long term benefits of angiotensin converting enzyme inhibitor therapy in patients with severe immunoglobulin A nephopathy: a comparison to patients receiving treatment with other antihypertensive agents and patients receiving no therapy. Am J Kidney Dis 1994; 23: 247-254.
- Mogensen CE, Keane WF, Bennett PH, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet 1995; 346: 1080-1084.
- Maschio G, Alberti D, Janin G, et al. Effect of angiotensin converting enzyme inhibitor benazapril on the progression of chronic renal insufficiency. N Engl J Med 1996; 334: 939-945.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, nondiabetic nephropathy. Lancet 1997; 349: 1857-1863.
- Ruggenenti P, Perna A, Gheradi G, et al. Renoprotective properties of ACE inhibition in nondiabetic nonnephrotic proteinuria. Lancet 1999; 354: 359-364.
- Statcorp. Stata statistical software, release 6.0. College Station (TX): Stata Corporation, 1999.
- Baker P, Hoy WE, Wang Z, et al. Towards evaluation of the cost-effectiveness of a treatment program for renal disease in Australian Aborigines. Presented at the 35th Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology; 1999 Mar 3-5; Brisbane (Qld).
- Mackenzie HS, Ziai F, Omer SA, et al. Angiotensin receptor blockers in chronic renal disease: the promise of a bright future. J Amer Soc Nephrol 1999; 10 Suppl 12: S283-S286.
- Mimran A, Ribstein J. Angiotensin receptor blockers: pharmacology and clinical significance. J Amer Soc Nephrol 1999; 10 Suppl 12: S273-S277.
- Hoy WE. Screening and treatment for renal disease: the community model. Nephrology 1998; 4 Suppl iii-iv: S90-S95.
- Minutes of the Inaugural Meeting of the National Aboriginal and Torres Strait Islander Renal Disease Scientific Working Group and its Guidelines Subcommittee. Office of Aboriginal and Torres Strait Islander Health. 1999; Nov 16 Alice Springs (NT).
(Received 14 Jul 1999, accepted 6 Apr 2000)
Authors' details Menzies School of Health Research, Darwin, NT.
Wendy E Hoy, FRACP, Senior Renal Consultant;
Philip R Baker, BSc, NHMRC PhD Student, Menzies School of Health Research, and Department of Social and Preventive Medicine, University of Queensland, Brisbane, QLD;
Angela M Kelly, RN, BAppSc, Senior Program Coordinator;
Zhiqiang Wang, PhD, Epidemiologist and Statistician, and Senior Research Officer.
Reprints will not be available from the authors.
Correspondence: Dr W E Hoy, Menzies School of Health Research, PO Box 41096, Casuarina, NT, 0811.
wendyATmenzies.su.edu.au
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Back to text | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Wendy E Hoy
- Philip R Baker
- Angela M Kelly
- Zhiqiang Wang
- 1.
- Cunningham J, Condon J. Premature mortality in Aboriginal adultsin the Northern Territory. Med J Aust 1996; 165: 309-312.
- 2.
- Spencer JS, Silva D, Hoy WE. An epidemic of renal failure amongAustralian Aborigines. Med J Aust 1998; 168: 537-541.
- 3.
- You J, Hoy W, Beaver C, Zhao Y. Costs of hemodialysis andhospitalisations for patients with end stage renal disease in the TopEnd of the Northern Territory. Presented at the 35th AnnualScientific Meeting of the Australian and New Zealand Society ofNephrology; 1999 Mar 3-5; Brisbane (Qld).
- 4.
- Jain SK, editor. Trends in mortality by causes of death inAustralia, the States and Territories during 1971-1992, and instatistical subdivisions during 1991-1992. Canberra: NationalCenter for Epidemiology and Population Health and Australian Bureauof Statistics, 1994. (ABS catalogue no. 3313.0)
- 5.
- Hoy WE, Pugsley DJ, Normal RJ, Hayhurst BG. A brief heath profile ofadults in a Northern Territory Aboriginal community: with anemphasis on preventable morbidities. Aust N Z J Public Health1997; 21: 121-126.
- 6.
- Hoy WE, Mathews JD, Pugsley DJ, et al. The multidimensional natureof renal disease: rates and associations of albuminuria in a high riskAboriginal community. Kidney Int 1998; 54: 1296-1304.
- 7.
- Cockcroft D, Gault MK. Prediction of creatinine clearance fromserum creatinine. Nephron 1976; 16: 31-41.
- 8.
- Hoy WE, Wang Z, Baker P, et al. The natural history of renal disease inan Australian Aboriginal community. Presented at the 35th AnnualScientific Meeting of the Australian and New Zealand Society ofNephrology; 1999 Mar 3-5; Brisbane (Qld).
- 9.
- McDonald S, Wang Z, Hoy WE. Physical and biochemical predictors ofdeath in an Australian Aboriginal cohort. Clin Exp PharmacolPhysiol 1999; 26: 618-621.
- 10.
- The Sixth Report of the Joint National Committee on Prevention,Detection, Evaluation and Treatment of High Blood Pressure (JNC VI).Arch Intern Med 1997; 157: 2413-2446.
- 11.
- Collins R, Peto R, MacMahon S. Blood pressure, stroke and coronaryartery disease. Part 2. Short term reductions in blood pressure:overview of randomised drug trials in their epidemiologicalcontext. Lancet 1990; 335: 827-838.
- 12.
- Lonn EM, Yusuf S, Jha P. Emerging role of angiotensin convertingenzyme inhibitors in cardiac and vascular protection.Circulation 1994; 90: 2056-2068.
- 13.
- Mogensen CE. Angiotensin converting enzyme inhibitors anddiabetic nephropathy. BMJ 1992; 304: 327-328.
- 14.
- Ravid M, Savin H, Lang R, et al. Proteinuria, renal impairment,metabolic control, and blood pressure in type 2 diabetes mellitus. A14-year follow up report on 195 patients. Arch Intern Med1992; 152: 1225-1229.
- 15.
- Ravid M, Savin H, Jutrin I, et al. Long term stabilizing effect ofangiotensin converting enzyme inhibition on plasma creatinine andon proteinuria in normotensive type 2 diabetic patients. AnnIntern Med 1993; 118: 577-581.
- 16.
- Gansevoort RT, de Zeeuw D, de Jong PE. Long term benefits of theantiproteinuric effect of angiotensin converting enzymeinhibition in nondiabetic renal disease. Am J Kidney Dis1993; 22: 202-206.
- 17.
- Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensinconverting enzyme inhibition on diabetic nephropathy. N Engl JMed 1993; 329: 1456-1462.
- 18.
- Bedogna V, Valvo E, Casagrande P, et al. Effect of ACE inhibition innormotensive patients with chronic glomerular disease and normalrenal function. Kidney Int 1994; 38: 101-107.
- 19.
- Cattran DC, Greenwood C, Ritchie S. Long term benefits ofangiotensin converting enzyme inhibitor therapy in patients withsevere immunoglobulin A nephopathy: a comparison to patientsreceiving treatment with other antihypertensive agents andpatients receiving no therapy. Am J Kidney Dis 1994; 23:247-254.
- 20.
- Mogensen CE, Keane WF, Bennett PH, et al. Prevention of diabeticrenal disease with special reference to microalbuminuria.Lancet 1995; 346: 1080-1084.
- 21.
- Maschio G, Alberti D, Janin G, et al. Effect of angiotensinconverting enzyme inhibitor benazapril on the progression ofchronic renal insufficiency. N Engl J Med 1996; 334: 939-945.
- 22.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici inNefrologia). Randomised placebo-controlled trial of effect oframipril on decline in glomerular filtration rate and risk ofterminal renal failure in proteinuric, nondiabetic nephropathy.Lancet 1997; 349: 1857-1863.
- 23.
- Ruggenenti P, Perna A, Gheradi G, et al. Renoprotectiveproperties of ACE inhibition in nondiabetic nonnephroticproteinuria. Lancet 1999; 354: 359-364.
- 24.
- Statcorp. Stata statistical software, release 6.0. CollegeStation (TX): Stata Corporation, 1999.
- 25.
- Baker P, Hoy WE, Wang Z, et al. Towards evaluation of thecost-effectiveness of a treatment program for renal disease inAustralian Aborigines. Presented at the 35th Annual ScientificMeeting of the Australian and New Zealand Society of Nephrology; 1999Mar 3-5; Brisbane (Qld).
- 26.
- Mackenzie HS, Ziai F, Omer SA, et al. Angiotensin receptorblockers in chronic renal disease: the promise of a bright future.J Amer Soc Nephrol 1999; 10 Suppl 12: S283-S286.
- 27.
- Mimran A, Ribstein J. Angiotensin receptor blockers:pharmacology and clinical significance. J Amer Soc Nephrol1999; 10 Suppl 12: S273-S277.
- 28.
- Hoy WE. Screening and treatment for renal disease: the communitymodel. Nephrology 1998; 4 Suppl iii-iv: S90-S95.
- 29.
- Minutes of the Inaugural Meeting of the National Aboriginal andTorres Strait Islander Renal Disease Scientific Working Group andits Guidelines Subcommittee. Office of Aboriginal and Torres StraitIslander Health. 1999; Nov 16 Alice Springs (NT).




