|
Research
Investigation of a cluster of leukaemia in the Illawarra region of New
South Wales, 1989-1996
Victoria J Westley-Wise, Bernard W Stewart, Irene Kreis, Paolo F
Ricci, Anthony Hogan, Chris Darling, Steve Corbett, John Kaldor,
Neill H Stacey, and Pauline Warburton
MJA 1999; 171: 178-183
For editorial comment, see Cartwright
Abstract -
Introduction -
Methods -
Results -
Discussion -
Conclusions -
Follow-up -
Acknowledgements -
References -
Authors' details
-
-
More articles on Haematology
|
Abstract |
Objectives: To investigate a cluster of leukaemia
among young people and assess the plausibility of a disease-exposure
relationship.
Design: Descriptive analysis of population-based
leukaemia incidence data, review of evidence related to the
causation of leukaemia, assessment of environmental exposures to
known leukaemogens, and resulting risks of leukaemia.
Setting: Illawarra region of New South Wales, Australia,
focusing on suburbs between the Port Kembla industrial complex and
Lake Illawarra (the Warrawong area).
Main outcome measures: Standardised incidence ratios
(SIRs) for leukaemia; current measured and past estimated ambient
air benzene concentrations; and expected leukaemia cases
attributable to estimates of ambient air benzene
concentrations.
Results: In 1989-1996, 12 leukaemia cases among
Warrawong residents aged less than 50 years were observed, more than
the 3.49 cases expected from the rate in the rest of the Illawarra
region (SIR, 343.8; 99% CI, 141.6-691.7). These people lived in
suburbs immediately to the south-southwest of a coke byproducts
plant (a major industrial source of benzene, one of the few known
leukaemogens). The greatest excess was among 15-24-year-olds (SIR,
1085.6; 99% CI, 234.1-3072.4). In 1996, ambient air concentrations
of benzene averaged less than 1 part per billion (ppb). Since 1970,
ambient air concentrations of benzene were estimated to have
averaged up to 3 ppb, about one-thousandth of the level at which
leukaemia risk has been identified in occupational epidemiological
studies. Using the risk assessment model developed by the US
Environmental Protection Agency, we estimate that past benzene
levels in the Warrawong area could have resulted in 0.4 additional
cases of leukaemia in 1989-1996.
Conclusions: The excess occurrence of leukaemia in the
Warrawong area in 1989-1996 is highly unusual. Current
environmental benzene exposure and the reconstructed past
environmental benzene exposure level are too low to explain the large
excess of leukaemia. The cause of the cluster is uncertain.
|
| | Introduction |
In July 1996 the Illawarra Public Health Unit (located in the
Wollongong/Port Kembla region, New South Wales) was notified that
four former students of a local high school had been diagnosed with
leukaemia since 1989. Preliminary investigations established that
a cluster of at least 11 people aged less than 40 years who had lived in
suburbs near the school had been diagnosed with leukaemia since 1989.
On the basis of New South Wales cancer registration data, only about 2
to 3 cases would have been expected.
Established causes of leukaemia include occupational benzene
exposure, ionising radiation, chemotherapeutic agents, and some
inherited and congenital conditions.1-5 Coke byproduct plants are
a recognised source of occupational, and potentially of
environmental, benzene exposures.1,6,7 The people in the
cluster lived in suburbs adjacent to the Port Kembla industrial
complex, which includes coke ovens and an associated byproducts
plant.
We report the investigation of the Illawarra region leukaemia
cluster and discuss the plausibility of a disease-exposure
relationship.
|
| |
Methods |
Our investigation followed published guidelines for cancer cluster
investigations.8-10 The main components were
evaluations of:
- the pattern of leukaemia incidence in the Illawarra
region, with specific attention to residential areas near the Port
Kembla industrial complex;
- environmental exposure to known and putative leukaemogens; and
- the plausibility of a disease-exposure relationship (whether past
environmental exposures to known leukaemogens could explain the
excess leukaemia occurrence).
The NSW Cancer Council Ethics Committee approved the study.
| |
Case finding and investigation | |
Active and passive case-finding methods were used to identify all
people resident in the Illawarra region (Wollongong, Shellharbour
and Kiama Local Government Areas) aged less than 50 years who had been
diagnosed with leukaemia in 1989-1996. The cut-off at age 50 years was
chosen as the index cases were young and the age interval 40-50 years
represents a natural change in leukaemia occurrence, when the
leukaemia risk begins to rise steeply.
We actively identified cases from bone marrow aspirate reports,
hospital discharge and day-only admission data, and discussions
with clinicians in Sydney and Wollongong, community members and
organisations. The population-based New South Wales Central Cancer
Registry provided the passive case-finding data.
For each leukaemia case, we sought to review the medical record and
interview the patient and/or a relative to obtain or confirm
information about dates of birth and diagnosis, leukaemia
cell-type, genetic and medical risk factors, residential and school
histories, and personal and/or parental occupational histories.
| |
Occurrence evaluation | |
Leukaemia incidence was analysed in eight areas within the Illawarra
region with a similar population size (about 20 000-30 000 in 1986),
including the area close to the Port Kembla industrial complex (Area 1) (Figure 1).
Leukaemia incidence rates in the whole Illawarra region, and each of
the eight areas within it, were compared with rates in a reference
population by calculating standardised incidence ratios (SIRs) as a
means of indirect age standardisation.11 The reference population
used was "Urban NSW" (Sydney, Wentworth, Central Coast,
Hunter and Illawarra administrative health areas). The calculation
of rates was based on place of residence at diagnosis.
Using Central Cancer Registry data, we calculated leukaemia SIRs for
males and females and for people aged less than 50 years for four
five-year periods which had Census years as their mid-points:
1974-1978, 1979-1983, 1984-1988 and 1989-1993.
The SIR is the ratio of the number of cancer cases in a study population
to the number of cases expected according to the age-specific rate in
the reference population (multiplied by 100).
For the Illawarra region, leukaemia rates could be calculated to
1996. Thus, for 1989-1996, leukaemia SIRs were calculated for each of
the Illawarra areas. In Area 1, they were also classified by age group
and cell-type (according to ICD-9),12 using the rest of the
Illawarra region as the reference population.
Australian Bureau of Statistics (ABS) census data for 30 June 1976,
1981, 1986 and 1991 were used for urban NSW reference
populations.13 For the Illawarra region,
the ABS provided population data by postcode.
Exact Poisson confidence intervals (CI) around the SIRs were
estimated.14 CIs were set at 99%, rather
than 95%, to reduce the possibility of identifying a chance excess of
cancer as statistically significant. SIRs and CIs were calculated
with SAS for Windows version 6.11. NSW Central Cancer Registry data
and urban NSW population data were accessed from NSW Health's
Health Outcomes Information and Statistical Toolbox, a
repository of health-related databases for New South Wales.
| |
Environmental monitoring and historical exposure reconstruction | |
We undertook an extensive review of the literature on risk factors for
and causes of leukaemia, and on carcinogenic effects of occupational
and environmental exposures.
The environmental assessment focused on exposure to known
leukaemogens from the 1970s to 1996. It involved interviewing
representatives from industry, government agencies, local
residents and workers; inspecting relevant sites; reviewing
government, industry and press reports; and collecting and
reviewing information on environmental and occupational exposure
for residents and workers.
The NSW Environment Protection Authority (EPA) and BHP Steel began
daily ambient air benzene monitoring in September 1996 in the
residential areas nearest the plant (Figure 2). Monitoring was also conducted at three control sites. The EPA
used the standard protocol developed by the US Environmental
Protection Agency (US EPA) for assessing toxic organic compounds in
ambient air.15 BHP used personal
samplers, adapted to a stationary role, which collected organic
vapours onto an active adsorbent medium by drawing air through the
sampler. Both the EPA and BHP analysed the samples with gas
chromatography at laboratories registered with the National
Association of Testing Authorities.
We estimated environmental benzene exposure from the main local
sources before September 1996. Using methods developed by the US
EPA,16 we estimated levels of
emissions from the coke production facilities for each year since
1970 (based on levels in 1996, adjusted for changes in plant equipment
and processes and changes in coke and benzene production).
Information related to benzene emissions from motor vehicles and
other petroleum sources, including Roads and Traffic Authority data
on local traffic volumes, was used to provide an upper estimate of the
extent to which emissions from these other major local sources may
have differed in previous years relative to 1996.
| |
Risk estimation |
The US EPA's benzene risk assessment model of
dose-response17 was used to estimate the
number of excess leukaemia cases expected in Area 1 between
1989-1996. It was assumed that the Area 1 population had breathed
ambient air (70 kg person breathing 20 m3 of air daily) with benzene
concentrations equivalent to the estimated maximum annual average
concentration since 1970 for the maximally exposed site,
continuously over a lifetime (24 hours per day for 70 years).
|
| |
Results |
| |
Case finding and investigation | |
The same cases were identified by both active and passive
case-finding methods. We identified 44 Illawarra residents aged
less than 50 years who were diagnosed with leukaemia in 1989-1996.
These included 12 people resident in Area 1 at diagnosis, and a 13th
person who had moved out of Area 1 a few months before diagnosis (Table 1).
None of these 13 people from Area 1 were found to have genetic or medical
risk factors for leukaemia. Nor had they ever worked in the production
of coke or its byproducts. Six were diagnosed with acute
lymphoblastic leukaemia (ALL), four with chronic myeloid leukaemia
(CML), and three with acute myeloid leukaemia (AML).
Immunophenotypic features and leukaemic classifications revealed
no unusual patterns. Six people have died.
Of the nine people from Area 1 aged 20 years or less, seven had lived
there all their lives, and two for about 11 years. Four attended the
same high school in the late 1980s, with three being in the same school
year; these three people had leukaemia of different cell-types.
| |
Occurrence evaluation | |
For the four five-year periods between 1974-1993, leukaemia
incidence in the Illawarra region, and each of its areas, was not
significantly different to that throughout urban New South
Wales.18 The incidence of total
cancers and other specific cancers (including lymphoma and multiple
myeloma) was also not significantly higher in Area 1.18 However, leukaemia incidence among Area 1 residents aged less than 50 years in 1989-1996 was more than three times higher than in the rest of
the Illawarra region (12 cases observed, versus 3.49 expected;
SIR, 344; P = 0.0003) (Table 2).
The SIR was more than 200 for all leukaemia cell-types, and was
significantly increased for ALL (Table 3).
The greatest excess of leukaemia was among teenagers and young
adults. Among 15-24-year-olds, five cases were observed, versus
0.46 expected (SIR, 1086; 99% CI, 234.1-3072; P =
0.0001).
| |
Environmental monitoring and reconstruction of historical
exposure | |
The Port Kembla industrial complex contains heavy industries such as
copper smelting, sulphuric acid and superphosphate manufacture,
petroleum depots, and Australia's largest steelworks, which
includes coke ovens and their byproducts plant. In spring and summer,
the predominant wind direction is from the northeast; hence, the
residential area where the people with leukaemia lived received the
greatest exposure from industrial emissions in the region.
Benzene was the only known leukaemogen for which local environmental
exposures may have been relevant. While ionising radiation is also an
established leukaemogen, and there is evidence that exposure to
occupational ethylene oxide or 1,3-butadiene can cause
leukaemia,3-5 no specific local
environmental sources of these agents were identified.
At the byproducts plant, coke oven gases are distilled into a benzene-
toluene-xylene commercial product (which is 80% benzene), while
past practices (until 1977) separated them. The closest residences
are more than 1 km from the plant, with all but two Area 1 cases residing
1-3 km from the plant. The other major benzene sources in Area 1 are
motor vehicles and petroleum storage tanks.
Both EPA and BHP monitoring found that ambient air benzene
concentrations in Area 1 averaged less than one part per billion (ppb)
in 1996 (Table 4), typical of urban sites in
Sydney.19 Analysis of benzene,
toluene and xylene ratios in the EPA's samples indicated that about
50% of the benzene was from petroleum.
Annual average ambient air benzene concentrations at the most
exposed site within Area 1, since 1970, are estimated to have been up to
about 3 ppb.
Roads and Traffic Authority data showed that traffic volumes in Area 1
have not changed appreciably since the 1970s.20 Local petroleum storage
tanks had a greater storage capacity in previous years, which may have
been associated with higher emissions.20 | |
Risk estimation |
The estimated maximum annual average ambient air benzene
concentration in Area 1 of 3 ppb is only about one-thousandth of
concentrations at which leukaemia risk has been detected in
occupational studies.1 The World Health
Organization (WHO) has concluded that occupational exposure to an
average of 1 part per million (1 ppm, ie 1000 ppb) over a working
lifetime has been associated with no statistical increase in
leukaemia deaths.1 Using the US EPA benzene risk assessment model,17 if a
population of 17 500 people (the number of people in Area 1 aged less
than 50 years in 1991) had all breathed air with an average benzene
concentration of 3 ppb over a lifetime, at the most 0.2 excess
leukaemia deaths (or 0.4 cases) would have been expected in
1989-1996.
|
| |
Discussion |
This investigation into the reported cluster found a highly
significant excess leukaemia occurrence in the Warrawong area,
particularly among teenagers, in 1989-1996. Estimated past
environmental benzene levels are too low to explain this excess.
| |
Cluster studies |
Cancer cluster studies have rarely provided insights into
aetiology.3,8-10,21,22 Studies of very
rare diseases with well-defined, high exposures are the most likely
to yield conclusive results.22 However, these
circumstances, in the context of geographic (spatial or
spatiotemporal) clusters, are uncommon.10,22 Disease clusters
occur continually in any population, and as such represent
"expectedly unexpected" events.9,22 Drawing boundaries tightly around people observed in clusters
inadvertently identifies and overestimates disease
excesses.21,22 However, in this
study, the geographic, age and time criteria setting the boundaries
were not defined or varied to influence the magnitude of the observed
excess. Area 1 was a natural geographic grouping of postcodes bounded
by industrial zones, Lake Illawarra and the Pacific Ocean. It
included suburbs and a postcode area in which no cases were resident.
The age range 0-49 years was broad given that the reported cluster was
among teenagers. On the other hand, the most recent period analysed
(1989-1996) was preceded by a period in which few leukaemia cases were
diagnosed.18
| |
Possible leukaemogens | |
Several known leukaemia causes and risk factors were excluded as
explanations for the cluster: ionising radiation, ethylene oxide,
1,3-butadiene, and genetic and medical risk factors. For other
agents considered, such as dioxins, pesticides, and heavy metals,
evidence is lacking of a causal relationship between these agents and
leukaemia, despite numerous studies conducted
worldwide.5 Although benzene is
structurally related to carcinogenic polycyclic hydrocarbons,
these agents are generally associated with lung and some other
cancers, but not leukaemia.23 Similarly, the influence
of genetic polymorphisms, specifically within the cytochrome P450
family, has been associated with lung cancer rather than
leukaemia.24 Viruses have been causally associated with the rare hairy cell
leukaemia and adult T-cell leukaemia.4,5 They have also been
suspected to cause leukaemia in childhood and adolescence, but there
is still little convincing evidence that they play an important
role.4,5,25
Several studies have associated parental
smoking with childhood leukaemia.3,26 While many other
parental occupational and/or personal exposures (including
pesticides, benzene, solvents, petroleum products, and spray
paints) have also been implicated in childhood leukaemia, most
relevant studies have used poor exposure measures, with
inconsistent results.2,3
To our knowledge no leukaemia "clusters" have been reported and
investigated in close proximity to industrial facilities similar to
those in the Warrawong area. Occupational exposure during steel and
coke production has been causally associated with an increased risk
of lung and certain other cancers, but not leukaemia.23,27-29 | |
Benzene |
Benzene was the only known human leukaemogen to which people in Area 1
had potentially significant environmental exposures. Despite the
inevitable focus on the steelworks and coke byproducts plant as a
benzene source, petrol exhaust and tobacco smoke (the primary source
of benzene for smokers, and relevant to non-smokers through passive
smoking) are the most significant sources in urbanised
populations.1 Food and water are not major
sources of benzene exposure,30 and the possibility of
contamination of the water supply in Area 1 with benzene from local
sources was examined and excluded.20 While other haematological malignancies have been associated with
occupational benzene exposure,31-36 the evidence has been
considered strongest for AML,1 which affected only three
people in this cluster. However, a recent review concluded that the
few available studies of leukaemia cell-types do not indicate larger
or more consistent elevations in risk for AML than for other
cell-types.36 | |
Study strengths and limitations | |
Studies of geographic cancer clusters must typically deal with poor
information about environmental exposures.22 In this study we had to rely
on estimates of past environmental benzene exposure, but several
factors suggest that the emission estimates for the coke production
facilities are accurate. An independent audit of the emissions
estimates concluded that the underlying assumptions were robust and
that the emissions inventory was calculated as accurately as
possible without an onsite testing program.37 The estimated past annual
average ambient air benzene concentration in the maximally exposed
part of the Warrawong area, 3 ppb, was similar to concentrations
measured38-40 and
modelled41 at similar distances from
byproducts plants overseas. Measured occupational benzene
exposures for Port Kembla byproducts plant workers between 1979 and
199642 were similar to
occupational monitoring results for byproducts plant workers from
the United States43 and Britain in the
1980s.29,44 Using the US EPA benzene risk assessment model,17 even if we
assume that average ambient air benzene concentration in Area 1 was 30
ppb (rather than the estimated 3 ppb), this level of exposure would
still only explain up to four excess leukaemia cases. Given that the
observed leukaemia excess was primarily among young people, the
default assumption of 70 kg adults used in our risk estimate is also
likely to have slightly overestimated the risk, and hence the number
of expected cases.
Consistent with public health principles, the US EPA benzene risk
assessment model, which uses data from studies of US rubber and
chemical workers,36,45,46 is itself based on
assumptions that would exaggerate rather than minimise risk,
including a linear relationship between exposure to genotoxic
carcinogens and leukaemia risk. However, results from some animal
studies suggest that a non-linear response may be more biologically
plausible.47,48 If this applies to
benzene-induced human leukaemia, application of the US EPA model may
considerably overestimate risks in the low exposure range.
More plausible are biologically based multistage stochastic models
for chemical carcinogenesis, which account for cellular birth,
death, initiation, promotion and other biological processes, each
stage being linked by a stochastic transition probability that
accounts for exposure (or dose).47,48 Such models are likely
to produce lower estimates of leukaemia risk for low levels of
environmental exposure.
However, large uncertainties are inherent in any conversion of risk
estimates from animal studies or occupational studies to risk
estimates for benzene exposure in the general community. Many people
-- children, people of reproductive age, those with other risk
factors -- may have susceptibilities to leukaemia quite different
from those of the male workers studied in occupational studies.
Animal and human studies have begun to clarify the potential risk
associated with relatively high transient and/or intermittent
benzene exposure49 versus cumulative
exposure, but the relationship is still poorly understood for low
dose extrapolations. In addition, people in the community are
exposed to a variety of agents, which may have as yet unidentified
additive and possibly synergistic effects.
|
| |
Conclusions |
On current knowledge, the recent ambient air benzene concentrations
in the Warrawong area represent a negligible leukaemia risk, and the
estimated past benzene concentrations are too low to explain the
large excess of leukaemia cases that occurred in 1989-1996. However,
factors such as variation in susceptibilities of individuals and
population groups such as children, the possible effects of
intermittent and high transient benzene exposures, and
interactions between different agents, mean that we cannot exclude a
causal association between leukaemia occurrence among young people
in the Warrawong area and chemical exposures.
|
| |
Follow-up |
A feasibility study is being undertaken to examine the potential for
relating disease to chemical exposures through a case-control
study. Broadly, chemical exposures of interest are personal
exposures (environmental and individual, to benzene and industrial
emissions in general) and parental exposures (focusing on those with
prior evidence of an association with leukaemia).
In addition, routine surveillance of leukaemia and lymphoma is
continuing, as is ambient air monitoring for benzene and other
hazardous pollutants in the Warrawong area.
|
Acknowledgements | |
We gratefully acknowledge the assistance given by the individuals
with leukaemia and their relatives, and other community members who
participated in the investigation's Community Reference Group. We
give special thanks to members of the Illawarra Leukaemia
Investigation Steering Committee for their commitment and
contributions: Giovanna Crocco and David Gilmour (Community
Reference Group), Richard Willison and Trevor Dunn (Illawarra
Public Health Unit), Joe Woodward and Craig Lamberton (NSW
Environmental Protection Authority), Ron Hales (Wollongong City
Council), and Christine Ewan (University of Wollongong). Many
individuals and organisations assisted in and supported the study,
but we would particularly like to thank the following organisations:
NSW Health; Illawarra Area Health Service; New South Wales Cancer
Council; BHP Port Kembla; NSW Environmental Protection Authority;
and the University of Wollongong. We also thank John Marthick
(University of Wollongong) and Paddy Ranasinghe (Illawarra Public
Health Unit) for preparing the maps.
|
| |
References |
- World Health Organization. Benzene (Environmental Health
Criteria No. 150). Geneva: International Programme on Chemical
Safety, 1993.
-
Ross JA, Davies SM, Potter JD, Robison LL. Epidemiology of
childhood leukaemia, with a focus on infants. Epidemiol Rev
1994; 16: 243-272.
-
Cartwright RA, Staines A. Acute leukaemias.Clin Haematol
1992; 5: 1-26.
-
Finch SC, Linet MS. Chronic leukaemias. Clin Haematol
1992; 5: 27-56.
-
Tomatis L, editor. Cancer: causes, occurrence and control (IARC
Scientific Publications No. 100). Lyon: International Agency for
Research on Cancer, 1990.
-
Fishbein L, O'Neill IK, editors. Benzene and alkylated
benzenes. Environmental carcinogens: methods of analysis and
exposure measurement, vol. 10 (IARC Scientific Publications No.
85). Lyon: International Agency for Research on Cancer, 1988.
-
United States Environmental Protection Agency. National
emission standards for hazardous air pollutants; regulation of
benzene; response to public comments. US Federal Register 1984, 40
CFR Part 61, AD-FRL-2523-7.
-
Centers for Disease Control. Guidelines for investigating
clusters of health events. MMWR Morb Mortal Wkly Rep 1990, 39
(R-11): 1-23.
-
Bender AP, Williams AN, Johnson RA, Jagger HG. Appropriate public
health responses to clusters: the art of being responsibly
responsive. Am J Epidemiol 1990; 132 Suppl 1: S48-S52.
-
Fiore BJ, Hanrahan LP, Anderson HA. State health department
response to disease cluster reports: a protocol for investigation.
Am J Epidemiol 1990; 132 Suppl 1: S14-S22.
-
Breslow NE, Day NE. Statistical methods in cancer
research. Vol II. Lyon: International Agency for Research on Cancer,
1987.
-
World Health Organization. Manual of the International
Statistical Classification of Diseases, Injuries and Causes of
Death. 9th Revision. Geneva: WHO, 1977.
-
Australian Bureau of Statistics. Estimated resident
populations by age and sex in statistical local areas, New South Wales
(Catalogue. No. 3209.1, 30 June 1976, 1981, 1986, 1991). Canberra:
ABS, 1978, 1983, 1988, 1993.
-
Daly L. Simple SAS macros for the calculation of exact binomial and
Poisson confidence limits. Comput Biol Med 1992; 22:
351-361.
-
United States Environmental Protection Agency. Compendium of
methods for the determination of toxic organic compounds in ambient
air. 2nd edition. Compendium method TO-14A. Determination of
volatile organic compounds (VOCs) in ambient air using specially
prepared canisters with subsequent analysis by gas chromatography
(EPA/625/R-96/010b). Cincinnati, OH: Center for Environmental
Research Information, 1999.
<http://www.epa.gov/ttn/amtic/files/ambient/airtox/to-14ar.pdf> Accessed 5 July 1999.
-
United States Environmental Protection Agency. AP-42, 5th ed.,
vol. I. Chapter 12: metallurgical industry. 12.2 Coke production
[draft]. Research Triangle Park, NC: Emission Factor And Inventory
Group, US EPA, 1995.
<http://www.epa.gov/ttn/chief/ap42pdf/c12s02.pdf>.
Accessed 5 July 1999. [No longer available, but see final version at <http://www.epa.gov/ttn/chief/ap42/ch12/final/c12s02.pdf>
Accessed 10 May 2001.
-
United States Environmental Protection Agency. Integrated risk
information system (IRIS). Benzene (CASRN 71-43-2). 16 October
1998. Cincinnati, OH: Environmental Criteria and Assessment
Office, Office of Health and Environmental Assessment, Office of
Research and Development, Cincinnati, 1996.
<http://www.epa.gov/ngispgm3/iris/subst/0276.htm>
Accessed 5 July 1999.
-
Westley-Wise V, Hogan A. Report on the occurrence of leukaemia
(1974-96) and other cancers (1974-93) in the Illawarra. Illawarra
Area Health Service. Wollongong 1997.
-
Wadge A, Salisbury J. Benzene. National Environmental Health
Forum Monographs, Air Series 2. National Environmental Health
Forum, South Australian Health Commission, Adelaide,1997.
-
Kreis I, Willison R. Environmental assessment related to a
leukaemia cluster. University of Wollongong. Wollongong 1997.
-
Olsen SF, Martuzzi M, Elliott P. Cluster analysis and disease
mapping -- why, when and how? A step by step guide. BMJ 1996;
313: 863-866.
-
Rothman KJ. A sobering start for the cluster busters'
conference. Am J Epidemiol 1990; 132 Suppl 1: S6-S13.
-
International Agency for Research on Cancer. Polynuclear
aromatic compounds. Part 3, Industrial exposures in aluminium
production, coal gasification, coke production, and iron and steel
founding. IARC Monographs on the evaluation of the carcinogenic risk
of chemicals to humans, vol. 34. Lyon: IARC, 1984.
-
Wormhoudt LW, Commandeur JNM, Vermeulen NPE. Genetic
polymorphisms of human N-acetyltransferase, cytochrome P450,
glutathione-S-transferase, and epoxide hydrolase enzymes:
relevance to xenobiotic metabolism and toxicity. Crit Rev
Toxicol 1999; 29: 59-124.
-
Alexander FE. Viruses, clusters and clustering in childhood
leukaemia: a new perspective? Eur J Cancer 1993; 29A:
1424-1443.
-
Sorahan T, Lancashire RJ, Hulten MA, Stewart AM. Childhood cancer
and parental use of tobacco: Deaths from 1953 to 1955. Br J
Cancer 1997; 75: 134-138.
-
Redmond CKA, Strobino BR, Cypress RH. Cancer experience among
coke byproduct workers. Ann N Y Acad Sci 1976; 217: 102-115.
-
Swaen GM, Slanged JAM, Volovics A, et al. Mortality of coke plant
workers in the Netherlands. Br J Indust Med 1991; 48: 130-135.
-
Hurley JF, Cherrie JW, Maclaren W. Exposure to benzene and
mortality from leukaemia: results from coke oven and other coal
product workers. Br J Indust Med 1991; 48: 502-504.
-
Wallace L. Environmental exposure to benzene: an update.
Environ Health Perspect 1997; 104: 1129-1136.
-
Yin SN, Hayes RB, Linet MS, Li GL, et al. A cohort study of cancer
among benzene-exposed workers in China: overall results. Am J
Indust Med 1996; 29: 227-235.
-
Hayes RB, Yin S-N, Dosemeci M, Li G-L, et al. Benzene and the
dose-related incidence of hematologic neoplasms in China. J Natl
Cancer Inst 1997; 89: 1065-1071.
-
Rinsky RA, Alexander B, Smith MD, Hornung R, et al. Benzene and
leukaemia: an epidemiological risk assessment. N Engl J Med
1987; 136: 1044-1050.
-
Christie D, Robinson K, Gordon I, Bisby J. A prospective study in
the Australian petroleum industry. I. Mortality. Br J Indust
Med 1991; 48: 507-510.
-
Wong O. An industry wide mortality study of chemical workers
occupationally exposed to benzene. II. Dose response analyses.
Br J Indust Med 1987; 44: 382-395.
-
Savitz DA, Andrews KW. Review of epidemiologic evidence on
benzene and lymphatic and hematopoietic cancers. Am J Indust
Med 1997; 31: 287-295.
-
Holmes Air Sciences 1997. Air quality report: review of BHP report
to the Illawarra Area Health Service Leukaemia Task Force. Report
prepared for the NSW Environment Protection Authority. Sydney:
Holmes Air Sciences, 1997.
-
Harkov R, Olsakovsky AC, Fillo JP. Determining the ambient
impacts of coke and coke by-products manufacturing on selected
pollutant levels in neighboring communities: I -- results from a
six-month ambient air benzene monitoring study. Air toxics and
volatile organic compounds: papers from the 84th Annual Meeting and
Exhibition of the Air and Waste Management Association, Vancouver,
1991. Vol 6. Pittsburgh: Air and Waste Management Association, 1991.
-
GDCh (Society of German Chemists) Advisory Committee on Existing
Chemicals of Environmental Relevance. Benzene. Weinhmam, VCH
Verlagsgesellschaft, 1988. In: World Health Organization. Benzene
(Environmental Health Criteria No. 150). Geneva: WHO, 1993: 36.
-
Fentiman AF, Neher MB, Kinzer GW, et al. Environmental monitoring
benzene (PB-295 641). Prepared for US EPA. Springfield, VA: Battelle
Columbus Laboratories, National Technical Information Service,
1979. In: IARC. Some industrial chemicals and dyestuffs (IARC
monographs on the evaluation of the carcinogenic risk of chemicals to
humans, vol. 29). Lyon: IARC, 1982.
-
United States Environment Protection Agency. Benzene emissions
from coke byproduct recovery plants -- background information for
proposed standards (EPA-450/3-83-016a). Research Triangle Park,
NC: Office of Air Quality Planning and Standards, 1984.
-
BHP Flat Products Division. Report to the Illawarra Area Health
Service. Port Kembla: BHP Flat Products Division, 1997.
-
Runion HE, Scott LM. Benzene exposure in the United States,
1978-1983: an overview. Am J Indust Med 1985; 7: 385-393.
-
Drummond L, Luck R, Afacan AS, Wilson HK. Biological monitoring of
workers exposed to benzene in the coke oven industry. Br J Indust
Med 1988; 45: 256-261.
-
Rinsky RA, Young RJ, Smith AB. Leukemia in benzene workers. Am J
Indust Med 1981; 2: 217-245.
-
Ott MG, Townsend DT, Fishbeck WA, Langner RA. Mortality among
workers occupationally exposed to benzene. Arch Environ
Health 1978; 33: 3-10.
-
Cox LA Jr, Ricci PF. Reassessing benzene cancer risks using
internal doses. Risk Analysis 1992; 12: 401-409.
-
Cox LA Jr, Ricci PF. Dose-response non-linearities for benzene
revisited: A reply to C Crump. Risk Analysis 1993; 14:
485-486.
(Received 2 July 1998, accepted 18 May 1999)
|
| | Authors' details |
Illawarra Public Health Unit, Illawarra Area Health Service,
Wollongong, NSW.
Victoria J Westley-Wise, MPH, FAFPHM, Director;
Anthony
Hogan, MSc(Hons), PhD, Public Health Officer.
Children's Cancer Research Institute, Sydney Children's Hospital,
Sydney, NSW.
Bernard W Stewart, PhD, FRACI, Research Director; now Head of
Cancer Control Program, South Eastern Sydney Area Health Service.
University of Wollongong, Wollongong, NSW.
Irene Kreis, PhD, FAFPHM, Senior Lecturer;
Paolo F Ricci,
MSc, PhD, Professorial Fellow.
BHP Steel Flat Products Division, Wollongong, NSW.
Chris Darling, MSc(Occup Med), FAFOM, Occupational Health
Advisor.
NSW Health Department, Sydney, NSW.
Steve Corbett, MPH, FAFPHM, Manager.
National Centre for HIV Epidemiology and Clinical Research,
University of New South Wales, Sydney, NSW.
John Kaldor, PhD, Deputy Director, and Professor of
Epidemiology.
University of Sydney, Sydney, NSW.
Neill H Stacey, BSc(Hons), PhD, Associate Professor.
Illawarra Regional Hospital, Illawarra Area Health Service,
Wollongong, NSW.
Pauline Warburton, MB BS, FRACP, Director.
Reprints: Dr V J Westley-Wise, Illawarra Public Health Unit,
PO Box 66, Keiraville, NSW 2500.
Email: vwestATdoh.health.nsw.gov.au
|
| | 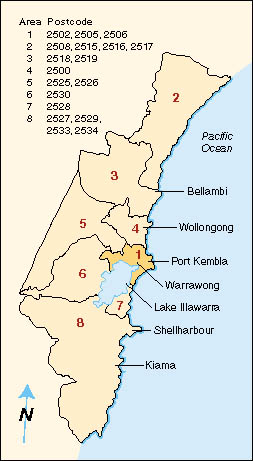 | | Figure 1 (above): Map of the Illawarra region, showing areas used for comparing leukamia incidence. |
| | Back to text | | | Figure 2 (below): Map of the Warrawong area (central portion of area 1), showing the location of the four ambient air monitoring stations. | 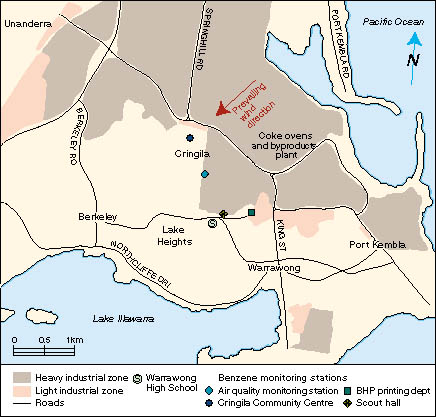 |
| | Back to text | | 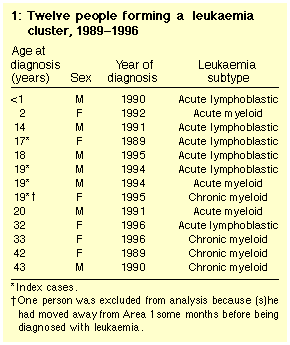 | | Back to text | | 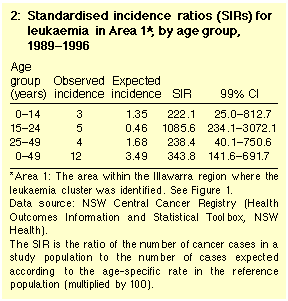 | | Back to text | | 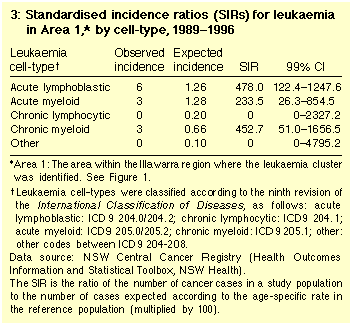 | | Back to text | | 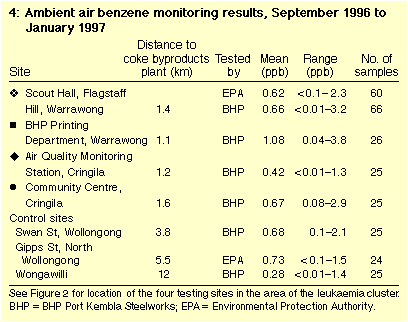 | | Back to text |
|










