Clinical record
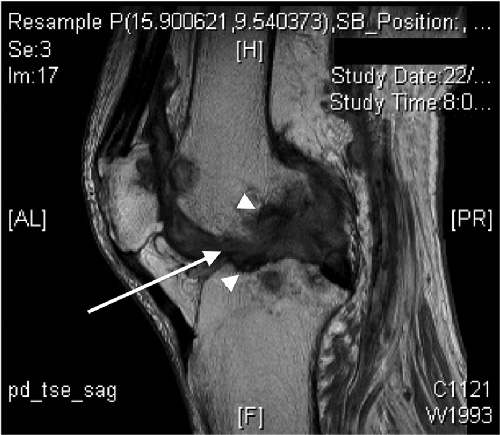
An 81-year-old non-English-speaking Filipino woman with type 2 diabetes mellitus presented with left knee pain and swelling of 18 months’ duration. Her mobility was restricted to less than 2 m because of pain. Synovial fluid obtained by needle aspiration during a recent visit to the Philippines had yielded a white cell count of 12 200 cells/μL (55% polymorphonucleocytes; reference interval [RI] not available) and no bacterial organisms. On her return to Australia, she was referred to an orthopaedic surgeon. Physical examination revealed chronically discharging wounds at previous aspiration sites of the medial and lateral aspects of the knee, which was swollen and tender but with good range of movement. The patient did not have a fever, and her C-reactive protein level was 18 mg/L (RI, < 10 mg/L)]. Magnetic resonance imaging demonstrated florid erosive synovitis, considered most consistent with rheumatoid or seronegative arthritis, although bone oedema was noted, suggesting osteomyelitis (Box 1). Culture of material from the sinuses for bacteria was negative.
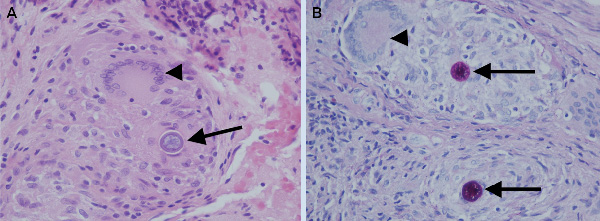
The first stage (debridement, spacer implantation and intravenous antibiotic therapy for 4 weeks) of a planned two-stage total knee replacement was performed. The patient received intraoperative and postoperative empirical treatment with vancomycin. Consultation with infectious diseases physicians was then sought. Microscopic examination of intraoperative specimens for bacteria, fungi and mycobacteria revealed no organisms. However, histopathological examination revealed numerous epithelioid granulomata with scattered multinucleated giant cells and a dense chronic lymphocytic inflammatory infiltrate. Some giant cells contained structures 20–100 μm in diameter, characteristic of fungal spherules in various stages of development. The spherules had slightly refractile rigid walls highly suggestive of Coccidioides species (Box 2).
Five intraoperative specimens inoculated on Sabouraud dextrose agar grew a white fluffy fungus on Day 2 of incubation. Sparse clinical specimen information, together with rapid colony growth meant Coccidioides spp were not suspected before histopathological diagnosis, and colonies were manipulated on open laboratory benches. Subsequent safety concerns about working with cultures of Coccidioides spp warranted killing of the organism with heat before further examination.Coccidioides immitis was identified by polymerase chain reaction (PCR) and DNA sequencing. Specifically, the D1-D2 domain of the fungal large subunit (28S) ribosomal RNA gene was amplified using published primers1 and amplicons sequenced by standard methodology. Sequences were subjected to BLASTn search2 and compared with archived fungal sequences in the United States National Institutes of Health GenBank database.
Expert opinion on management of exposed laboratory staff3 indicated that the risk in this situation was low (colonies were immature and exposure minimal), but warranted prophylaxis. Laboratory staff exposed to the fungus were monitored for symptoms, prescribed 6 weeks of itraconazole 200 mg twice daily for prophylaxis,3 and had immunodiffusion serology performed (in-house) before and after prophylaxis. All returned negative results.
The operative wound healed well, clinical evidence of infection resolved and the patient became ambulant. Her trough serum itraconazole levels, initially low at 478 μg/L (target, > 500 μg/L4), increased to 977 μg/L with a dosage of 300 mg twice daily. After 4 weeks of therapy, itraconazole toxicity occurred (3 325 μg/L [maximum trough therapeutic level, < 2000 μg/L4]) manifested by anorexia and hypokalaemia (serum potassium, 2.6 mmol/L [RI, 3.2–5.0 mmol/L]) and was successfully managed with dose reduction and ongoing therapeutic drug monitoring. Neither toxicity nor evidence of infection has recurred after 9 months of long-term suppressive itraconazole therapy.
Imported pathogens are increasingly recognised as potential biosecurity concerns, but may be overlooked when the clinical syndrome and causative organism are unexpected. This case highlights the need to consider an infectious aetiology in an older migrant with a chronic inflammatory monoarthritis. It emphasises the need for good communication between laboratory disciplines, and demonstrates preliminary success with a combined surgical–medical–pathology–pharmacological approach.
Coccidioidomycosis (“pseudotuberculosis”) is an invasive fungal infection caused by Coccidioides species (C. immitis and C. posadasii). It is not autochthonously acquired in Australia, being endemic to western-hemisphere deserts, primarily in the south-west of the United States, and Mexico. After respiratory inoculation, arthroconidia (hyphal forms) transform into multinucleated spherules, which rupture, propagating new spherules.5 Crucial diagnostic information obtained through the histopathological detection of spherules in this patient (Box 2) was rapidly relayed to the microbiology laboratory.
Coccidioides spp are classified as Risk Group 3 pathogens in Australia (as is Bacillus anthracis).6 Analytical work performed on these pathogens must be carried out in a biological safety cabinet in a physical containment level 3 sealed laboratory with negative air pressure.6 As one of the ten most common laboratory-acquired infections,7 with laboratory attack rates exceeding those of natural exposure,8 travel history to an endemic area is crucial information for laboratory staff.3,9 Suspected C. immitis colonies take up to 5 days to manifest with “typical” morphological characteristics. Further, risk to laboratory staff may be longstanding if hardy arthroconidia disseminate. Extensive and costly cleaning measures are then required.
In our patient, it is highly likely that infection was acquired in the south-west US about 18 months earlier, accounting for her pneumonic illness, which occurs in 40% of infected immunocompetent patients.10 A thorough history including travel should be obtained; for non-English-speaking patients, this should be through a professional interpreter. The history of residence in the US might have raised suspicion of coccidioidomycosis sooner. Our patient’s ethnicity and age might have facilitated subsequent osteoarticular spread.10 Although less than 5% of infected patients develop dissemination, it is significantly more likely in Filipino people, possibly related to the HLA class II DRB1*1301 allele, or blood group.10 Diabetes mellitus is not a recognised risk factor for dissemination.
Common extrapulmonary sites of infection are bone, joint, skin and brain. The knee is the most common osteoarticular site of infection.8,10 Failure to grow the organism from initial knee aspirates may have been because of sequestered organisms in the synovium or bone, or the failure to request fungal cultures. Prolonged diagnostic delay led to advanced chronic arthritis. The presence of multiple discharging sinuses, relatively low C-reactive protein level and negative bacterial cultures in a native knee, combined with this patient’s ethnicity and age, should have raised suspicion of an atypical infective process (eg, mycobacterial, fungal). Differential diagnoses include non-infective erosive arthritides (rheumatoid, seronegative, gout). Preoperative diagnosis could have permitted earlier adjunctive antifungal therapy.
Aggressive debridement and removal of infected synovium is recommended for optimal outcomes.8 In this case, a modified two-stage replacement arthroplasty was undertaken. As incomplete surgical debridement was suspected, long-term suppressive antifungal therapy was continued.
Itraconazole was used because of its efficacy against C. immitis11 and its superiority over fluconazole in skeletal infection.8 As this case shows, the pharmacokinetics of itraconazole are complex, and therapeutic drug monitoring is recommended to optimise dosing.4,8,11 Toxicity requiring discontinuation is rare (4%); nausea or vomiting is the most common adverse event (10%), and hypokalaemia occurs in about 6% of patients.12 In our patient, toxicity developed after subtherapeutic steady-state trough levels prompted a dose increase. Variable absorption and the long half-life (20–30 hours) may also have contributed to dosing difficulties.
Fluconazole, voriconazole or posaconazole are second-line options.11 Amphotericin formulations are used for serious infections such as meningitis.11 Susceptibility testing is not routinely performed, to limit handling of the organism5 and because susceptibility is predictable.
Lessons from practice
In an older migrant, infection should be strongly considered as part of the differential diagnosis for inflammatory monoarthritis, even when initially culture-negative.
Travel history is critically important to both clinicians and diagnostic laboratory staff.
Prompt communication and collaboration between pathology laboratories can expedite diagnosis and mitigate hazard.
Therapeutic drug monitoring of itraconazole is required due to its complex pharmacokinetics and bioavailability.
- 1. Clinical and Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. Wayne, PA: National Committee for Clinical Laboratory Standards, 2008. (CLSI document MM18-A.) http://www.clsi.org/source/orders/free/MM18-a.pdf (accessed Nov 2011).
- 2. Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25: 3389-3402.
- 3. Stevens DA, Clemons KV, Levine HB, et al. Expert opinion: what to do when there is Coccidioides exposure in a laboratory. Clin Infect Dis 2009; 49: 919-923.
- 4. Poirier JM, Cheymol G. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 1998; 35: 461-473.
- 5. Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci 2007; 1111: 301-314.
- 6. Standards Australia Limited, Standards New Zealand. Safety in Laboratories part 3: microbiological safety and containment: AS/NZS 2243.3. Sydney: SAI Global Limited, 2010.
- 7. Singh K. Laboratory-acquired infections. Clin Infect Dis 2009; 49: 142-147.
- 8. Blair JE. State-of-the-art treatment of coccidioidomycosis skeletal infections. Ann N Y Acad Sci 2007; 1111: 422-433.
- 9. Chaturvedi V, Ramani R, Gromadzki S, et al. Coccidioidomycosis in New York State. Emerg Infect Dis 2000; 6: 25-29.
- 10. Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore) 2004; 83: 149-175.
- 11. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis 2005; 41: 1217-1223.
- 12. Tucker RM, Haq Y, Denning DW, Stevens DA. Adverse events associated with itraconazole in 189 patients on chronic therapy. J Antimicrob Chemother 1990; 26: 561-566.





We thank Dr John Ray, Principal Hospital Scientist, Division of Clinical Pharmacology and Toxicology, St Vincent’s Hospital, Sydney for assistance with itraconazole levels. Anna Ralph is supported by National Health and Medical Research Council Training Fellowship APP1016567 and a Sydney Medical School Foundation grant to the Sydney Emerging Infections and Biosecurity Institute.
No relevant disclosures.