The known: According to current guidelines, repeat liver transplantation is not routinely considered because of poorer recipient survival and the shortage of donor organs.
The new: Recipient and graft survival (1–15 years) are similar for children receiving first or subsequent liver transplants. The routine use of split liver grafts in children has alleviated the shortage of donor organs and markedly reduced waiting list mortality.
The implications: Given the excellent survival following liver retransplantation in children and the increased availability of donor organs, repeat liver transplantation in children is justified on both medical and ethical grounds.
Outcomes for children who have received liver transplants have improved considerably in recent years, but outcomes following liver retransplantation have not been reported in detail.1 The limited data available indicate that patient and graft survival after retransplantation are poorer than after primary liver transplantation.2,3,4,5,6,7 A 2002 British study found that 1‐ and 5‐year patient survival after first liver transplantation in children were respectively 71.7% and 64.7%, compared with 65.6% and 56.7% after retransplantation.4 In the past, the shortage of donor organs has caused significant waitlist mortality for children needing liver transplants (7–12% in the United States [2016],8 6.4% in Australia [2007]9). In response, donor allocation protocols in Australia and New Zealand were revised in 2004, so that organs from donors under 18 years of age are now offered first to children on the waiting list, with those in intensive care receiving priority.10
In 1999, the authors of a landmark article on guidelines for selecting patients for liver transplantation recommended that retransplantation should not be routinely considered.11 Some ethicists have proposed that potential recipients be prioritised exclusively according to urgency and the likelihood of 5‐year survival.12 Others have argued that prior transplantation should be considered when allocating donor organs to reduce the frequency of some recipients receiving multiple transplants while others die waiting for organs.13
Prognostic models have indicated that the need for life support, use of split liver grafts, neonatal or familial cholestasis, paucity of bile ducts, and congenital abnormalities are associated with poor outcomes for children after liver retransplantation.14 Split liver grafting was first used in children in Australia and New Zealand in 1989 to maximise the number of patients receiving transplants,15 and since 2002 optimal donor livers have been split whenever possible.10 However, some early studies reported poorer outcomes for recipients of split grafts;5,16 one found that split liver transplantation reduced waiting time, but was associated with reduced graft survival.17 In contrast, the Australia and New Zealand Liver Transplant Registry (ANZLTR) has reported that split liver graft survival is similar to that of whole organs, and significantly better than for reduced size grafts in children.18 Other groups have since reported similar findings. In the United Kingdom, the Queen Elizabeth University and Birmingham Children's Hospital adopted an “intention to split” policy for first transplantations in both children and adults; it was found that graft and patient survival after first transplantations were similar for split and whole grafts, and that waitlist mortality for children was almost eliminated.19
For more than 30 years, the prospective ANZLTR has collected comprehensive data on liver transplantation in children and adults, including information on donors, recipients and medical variables, from all transplantation services in Australia and New Zealand. Child recipients are later transitioned to adult transplantation services, facilitating long term follow‐up. ANZLTR data are de‐identified and updated annually.18
Our aim was to assess long term graft and patient survival after donor liver retransplantation in children in Australia and New Zealand during 1986–2017, and to determine the factors that influence survival. We also assessed whether graft type and causes of graft failure leading to retransplantations have changed over time.
Methods
All Australian and New Zealand children (under 18 years of age) who underwent liver retransplantation during 1986–2017, in all four paediatric and six adult liver transplantation centres, were included in our retrospective cohort analysis. All patients were followed until graft failure or patient death, in some cases past the age of 18.
Data extraction
We extracted ANZLTR data for the primary endpoints (graft and patient survival). We also extracted data on:
- Recipient variables: age (and dates of birth and death), sex, weight, era of transplant, number of grafts, serum biochemistry, and retransplantation interval. Recipient Paediatric End‐Stage Liver Disease (PELD) score — a measure of the severity of liver failure based on biochemical parameters; higher positive values indicate more severe liver failure20 — was also collected.
- Cause of graft failure: primary non‐function, hepatic artery or portal vein thrombosis, biliary causes, recurrent liver disease, acute rejection, or chronic rejection.
- Donor variables: age, sex, weight, requirement for air travel, cold ischaemic time, and graft type (split, reduced size, whole).
Sex, age, weight, and PELD score were continuous variables, while retransplantation interval, number of transplants, type of graft, and air travel were categorical. The ANZLTR dataset contains records for each liver graft; data for patients who had more than one retransplantation are presented by individual graft in the analysis set. We compared outcomes for 1986–2000 with those for 2001–2017.
Statistical analysis
Variables are summarised as medians with interquartile ranges (IQRs), and differences were assessed in Mann–Whitney U tests. Graft and patient survival were estimated in Kaplan–Meier curves, and groups compared in log‐rank tests. Univariate and multivariate Cox regression analysis included all variables except cold ischaemic time, PELD score, and serum biochemistry, the availability of data for which was inadequate for this analysis. All analyses were performed in SPSS 23; P < 0.05 (two‐sided) was deemed statistically significant.
Ethics approval
The Liver and Intestinal Transplant Advisory Committee of the Transplantation Society of Australia and New Zealand approved the study; the University of Western Australia Human Research Ethics Committee exempted the study from formal ethics approval (reference, RA/4/20/6327).
Results
A total of 933 liver transplantations were performed in children in Australia and New Zealand during 1986–2017, including 142 retransplantations (15%), six from live donors and 136 from deceased donors. During 1986–2000, 59 retransplantations were performed, and 83 during 2001–2017. The median time between first and subsequent transplantation was 0.2 years (IQR, 0.03–1.4 years) during 1986–2000, and 1.8 years (IQR, 0.1–6.8 years) during 2001–2017 (P = 0.002). The median age of recipients was 4 years (IQR, 1–8 years) during 1986–2000 and 9 years (IQR, 4–12 years) during 2001–2017 (P = 0.001). Complete laboratory data for children receiving second transplants before 2000 were not available; for recipients during 2001–2017, the median PELD score was 4.4 (IQR, –1.8 to 12.8 years; range, –14.6 to 28.3 years) (Box 1).
Kaplan–Meier survival analysis indicated that survival was significantly greater during 2001–2017 than 1986–2000 (P < 0.001). During 2001–2017, graft survival one year after retransplantation was 84%, at 5 years 75%, at 10 years 70%, and at 15 years 54%; patient survival was 89% at one year, 87% at 5 years, 87% at 10 years, and 71% at 15 years (Box 2). Forty‐six of 59 retransplantation grafts (78%) during 1986–2000 failed; in 13 cases (28%) the patients received third liver transplants. Twenty‐four of 83 retransplantation grafts during 2001–2017 failed (29%); 11 recipients (46%) received third transplants.
In univariate analyses, retransplantation period significantly influenced graft survival (2001–2017 v 1986–2000: hazard ratio [HR], 0.37; 95% confidence interval [CI], 0.22–0.61), as did donor age (per year: HR, 1.02; 95% CI, 1.01–1.04) and donor weight (per kg: HR, 1.27; 95% CI, 1.06–1.51), but not patient age, sex or weight, total number of grafts, graft type, cause of graft failure, time between transplants, donor sex or requirement for air travel (online Supporting Information).
In separate analyses by retransplantation period, donor age was significantly associated with improved graft survival during 2001–2017 in both univariate and multivariate analyses; recipient weight was significantly associated with improved graft survival for retransplantations during 1986–2000 (Box 3). The total number of grafts (Box 4) and type of graft (Box 5) did not influence graft survival during either period.
A total of 124 of 142 retransplantation patients (87%) were followed beyond 18 years of age, 13 of whom (10%) experienced graft failure as adults. Eight of these patients died without further retransplantation (median age, 28 years; IQR, 23–33 years; median graft survival, 18.6 years; IQR, 13–20 years); five received further liver grafts at a median age of 23 years (IQR, 20–24 years; median graft survival, 9.8 years; IQR, 9.2–12 years).
Graft failure: causes and graft type
The proportion of graft failures leading to retransplantation attributed to hepatic artery thrombosis or portal vein thrombosis was smaller in 2001–2017 than 1986–2000 (29% v 53%), as was that of graft non‐function (4% v 17%) (Box 6). The proportions of graft failures that involved whole grafts (33 of 83, 40% v 18 of 59, 30%) or split grafts (35 of 83, 42% v 10 of 59, 17%) were larger during 2001–2017 than 1986–2000, and that of reduced sized grafts consequently smaller (15 of 83, 18% v 31 of 59, 53%). During 2001–2017, one of 33 graft failures following whole graft transplantation (3%) was attributed to graft non‐function, compared with 3 of 15 with split (20%) and 7 of 35 with reduced size grafts (20%) (Box 7).
Survival for children and adults
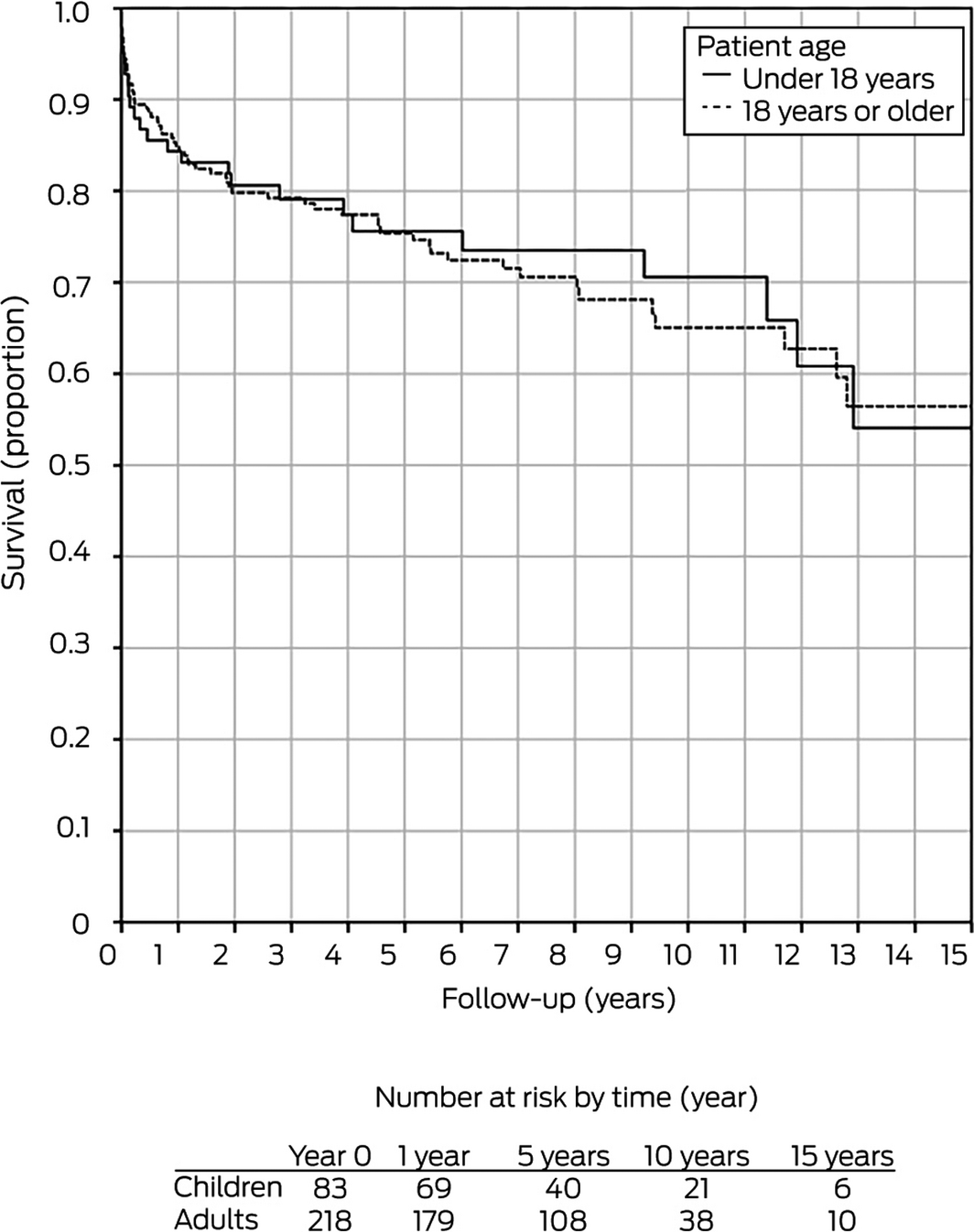
During 2001–2017, one‐year graft survival for adults following liver retransplantation was 85%, 75% at 5 years, 64% at 10 years, and 53% at 15 years; patient survival was 89% at one year, 81% at 5 years, 74% at 10 years, and 64% at 15 years.21 These rates were not significantly different from those for children undergoing liver retransplantation (Box 8).
Discussion
We report the first registry‐based study of the long term outcomes of liver retransplantation in children undertaken over a period of 30 years. The ANZLTR database allows long term graft and patient survival to be followed into adulthood, whereas other reported studies followed recipients only until they were 18 years old, to the transition from paediatric to adult transplantation care.3,14 Further, since 2002 it has been the policy in Australia and New Zealand to split optimal donor livers, increasing the use of split liver grafts during 2000–2017.
We found that patient survival — 1 year, 89%; 5 years, 87%; 10 years, 87%; 15 years, 71% — and graft survival — 1 year, 84%; 5 years, 75%; 10 years, 70%; 15 years, 54% — following liver retransplantation in children during 2001–2017 were excellent, and that they were markedly higher than following retransplantations during 1986–2000. The 2001–2017 rates are comparable with the pooled patient survival for all children in Australia and New Zealand receiving liver transplants during this period (1 year, 94%; 5 years, 89%; 10 years, 88%; 15 years, 83%).18 Patient and graft survival following liver retransplantation were similar for children and adults in Australia and New Zealand during 2000–2017, and graft survival was excellent compared with aggregate rates reported by United States transplantation registry studies covering 1989–2006 (Box 9).3,14
A major factor that influences overall retransplantation survival is whether children with graft failure receive additional liver transplants, as indicated by the fact that patient survival is higher than graft survival (Box 2). Second retransplantations followed 11 of 24 retransplantation graft failures during 2001–2017 (46%), and this contributed to excellent patient survival.
Factors reported to be associated with poorer outcomes for children after liver retransplantation include being on life support at the time of retransplantation, having neonatal or familial cholestasis, paucity of bile ducts, congenital abnormalities, and receiving a split liver graft.14 In contrast, we found that only donor age significantly influenced graft survival during 2001–2017. Moreover, the median PELD score during this period was 4.2, suggesting that most recipients were not critically ill at the time of retransplantation, which would have a positive impact on recipient survival.23 A PELD score of greater than 20 is associated with increased waitlist mortality and critical illness.9 One potential reason for the low PELD scores is that the routine use of split liver grafts in Australia and New Zealand reduces waitlist time for children without disadvantaging adult recipients. Waitlist mortality during 2017 was 1.4%,18 and under the British intention‐to‐split policy there were no waiting list deaths of children during 2011–2014.19 The use of split grafts in Australia and New Zealand for retransplantation was not associated with higher rates of graft loss; graft survival was similar for split, whole liver, and reduced size grafts.
A further factor that may have contributed to the excellent 15‐year outcomes is the management of transition of care to adult services. Non‐adherence with medical follow‐up and medications is common among adolescents, including during the transition to adult transplantation services.24 A recent Australian study of the transition of liver transplant recipients from paediatric to adult care found high rates of medication adherence and clinic attendance.25
Limitations
Our study shared the limitations common to all registry‐based analyses. The data available for analysis were for variables collected for the ANZLTR database. Data were not available for all recipients for all variables; laboratory data were less complete for retransplantations prior to 2000. Data on delisted or relisted patients were not available, but children on the waiting list with critical illness are generally given priority and are not delisted. The sample size restricted our statistical analysis; in particular, subgroup analyses by cause of initial graft failure or disease recurrence, and assessment of outcomes for specific age groups were not possible because of low subgroup numbers. Non‐compliance with prescribed treatment may contribute to some cases of chronic organ rejection, but data on compliance were not available. Our study relied on accurate reporting of data to the ANZLTR by the participating liver transplantation services.
Conclusion
We found that 15‐year patient and graft survival for children following liver retransplantation during 2001–2017 were excellent. Further, outcomes for patients in Australia and New Zealand receiving split liver grafts are similar to those for children receiving other graft types. Finally, graft and patient survival for the few children requiring multiple retransplantations were similar to those undergoing retransplantation only once. Our findings challenge views of the relative priority of children requiring first or subsequent liver transplants. The routine use of split liver grafts in Australia and New Zealand has increased the supply of donor grafts for candidate recipients and reduced waiting list mortality. Accordingly, split liver grafts should be used for both first and subsequent transplantations in children, and organ allocation should be based solely on need, not on the number of grafts the child has previously received.
Box 1 – Recipient and donor characteristics for 142 liver retransplantations in children in Australia and New Zealand, 1986–2017
|
Characteristic |
All |
1986–2000 |
2001–2017 |
P |
|||||||||||
|
|
|||||||||||||||
|
Recipients |
|
|
|
|
|||||||||||
|
Number of transplantations |
142 |
59 |
83 |
|
|||||||||||
|
Age (years*), median (IQR) |
7 (2–11) |
4 (1–8) |
9 (4–12) |
0.001 |
|||||||||||
|
Sex (boys) |
61 (43%) |
23 (39%) |
38 (46%) |
0.42 |
|||||||||||
|
Retransplantation interval (years), median (IQR) |
0.8 (0.03–3.7) |
0.2 (0.03–1.4) |
1.8 (0.1–6.8) |
0.002 |
|||||||||||
|
Weight (kg), median (IQR) |
20.0 (11.0–32.4) |
14.3 (10.0–25.5) |
25.6 (11.0–32.4) |
0.002 |
|||||||||||
|
PELD, median (IQR) |
— |
NA |
4.4 (–1.8 to 12.8) |
— |
|||||||||||
|
Creatinine (μmol/L), median (IQR) |
— |
NA |
51 (30–68) |
— |
|||||||||||
|
Sodium (mmol/L), median (IQR) |
— |
NA |
138 (135–140) |
— |
|||||||||||
|
Total number of transplants |
|
|
|
0.81 |
|||||||||||
|
Two (first retransplantation) |
124 (87%) |
52 (88%) |
72 (87%) |
|
|||||||||||
|
Three (second retransplantation) |
18 (13%) |
7 (12%) |
11 (13%) |
|
|||||||||||
|
Donors |
|
|
|
|
|||||||||||
|
Sex (boys) |
81 (57%) |
37 (63%) |
44 (53%) |
0.10 |
|||||||||||
|
Age (years), median (IQR) |
27 (17–38) |
26 (19–40) |
27 (17–37) |
0.80 |
|||||||||||
|
Weight (kg), median (IQR) |
67 (50–75) |
70 (60–75) |
65 (50–78) |
0.80 |
|||||||||||
|
Type of graft used |
|
|
|
0.002 |
|||||||||||
|
Whole liver graft |
51 (36%) |
18 (30%) |
33 (40%) |
|
|||||||||||
|
Split liver graft |
45 (32%) |
10 (17%) |
35 (42%) |
|
|||||||||||
|
Reduced size graft |
46 (32%) |
31 (53%) |
15 (18%) |
|
|||||||||||
|
Cold ischaemic time (min), median (IQR) |
— |
NA |
403 (304–568) |
— |
|||||||||||
|
Air transport needed |
53 (37%) |
28 (47%) |
25 (30%) |
0.023 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; NA = not available; PELD = Paediatric End‐Stage Liver Disease score. * Rounded to nearest year. |
|||||||||||||||
Box 2 – Liver retransplantation in children, 1986–2017: graft and patient survival, by retransplantation period
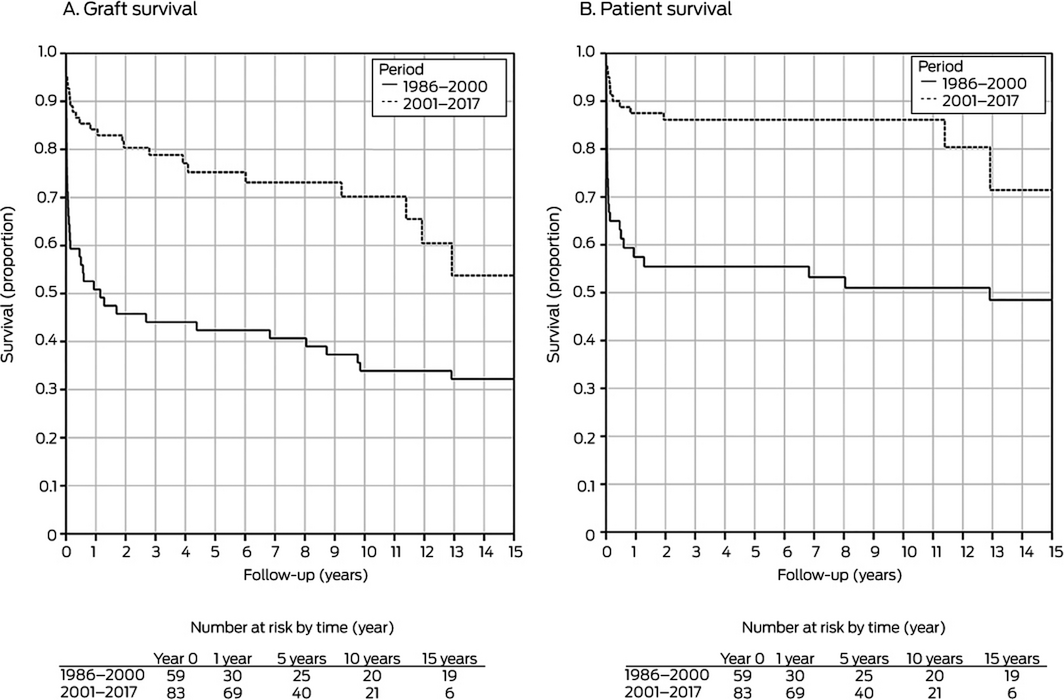
For both graft and patient survival, 1986–2000 v 2001–2017: P < 0.001.
Box 3 – Graft survival following liver retransplantation in children, 1986–2017: univariate and multivariate analyses
|
|
1986–2000 |
2001–2017 |
|||||||||||||
|
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||||||||
|
Hazard ratio (95% CI) |
Adjusted hazard ratio* (95% CI) |
Hazard ratio (95% CI) |
Adjusted hazard ratio* (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Recipient characteristics |
|
|
|
|
|||||||||||
|
Sex |
|
|
|
|
|||||||||||
|
Boys |
1 |
1 |
1 |
1 |
|||||||||||
|
Girls |
1.81 (0.97–3.38) |
2.15 (0.94–4.91) |
1.00 (0.67–1.50) |
1.10 (0.68–1.78) |
|||||||||||
|
Age, per year |
0.99 (0.93–1.05) |
1.27 (0.99–1.62) |
1.00 (0.92–1.08) |
0.98 (0.77–1.24) |
|||||||||||
|
Weight, per kg |
0.99 (9.75–1.01) |
0.91 (0.83–0.99) |
1.00 (0.98–1.02) |
1.01 (0.95–1.07) |
|||||||||||
|
Retransplantation interval |
|
|
|
|
|||||||||||
|
< 7 days |
1 |
1 |
1 |
1 |
|||||||||||
|
7–30 days |
1.36 (0.64–2.89) |
2.61 (0.93–7.32) |
0.93 (0.32–2.77) |
0.79 (0.14–4.53) |
|||||||||||
|
> 30 days |
1.01 (0.49–2.09) |
1.52 (0.58–3.97) |
1.25 (0.29–5.42) |
0.14 (0.01–2.00) |
|||||||||||
|
Total number of transplantations |
|
|
|
|
|||||||||||
|
Two |
1 |
1 |
1 |
1 |
|||||||||||
|
Three |
0.58 (0.26–1.31) |
0.62 (0.25–1.52) |
2.07 (0.48–8.86) |
0.72 (0.14–3.67) |
|||||||||||
|
Donor characteristics |
|
|
|
|
|||||||||||
|
Sex |
|
|
|
|
|||||||||||
|
Male |
1 |
1 |
1 |
1 |
|||||||||||
|
Female |
1.18 (0.28–5.00) |
0.86 (0.30–2.48) |
0.82 (0.35–1.90) |
0.70 (0.24–2.02) |
|||||||||||
|
Age, per year |
1.02 (1.00–1.03) |
1.01 (0.97–1.04) |
1.03 (1.01–1.06) |
1.05 (1.01–1.09) |
|||||||||||
|
Weight, per kg |
1.01 (0.99–1.03) |
1.02 (0.98–1.06) |
1.02 (0.99–1.04) |
1.00 (0.97–1.03) |
|||||||||||
|
Type of graft |
|
|
|
|
|||||||||||
|
Whole liver graft |
1 |
1 |
1 |
1 |
|||||||||||
|
Split liver graft |
0.71 (0.36–1.38) |
2.04 (0.37–11.2) |
1.37 (0.36–5.19) |
2.35 (0.45–12.2) |
|||||||||||
|
Reduced size graft |
0.95 (0.43–2.11) |
0.85 (0.33–2.21) |
2.32 (0.65–8.25) |
2.42 (0.41–14.3) |
|||||||||||
|
Air transport |
|
|
|
|
|||||||||||
|
Yes |
1 |
1 |
1 |
1 |
|||||||||||
|
No |
1.83 (0.99–3.41) |
1.64 (0.77–3.47) |
1.97 (0.66–5.88) |
1.67 (0.51–5.50) |
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Adjusted for all other included variables. |
|||||||||||||||
Box 4 – Graft survival following liver retransplantation in children, 1986–2017, by number of grafts received and transplantation period
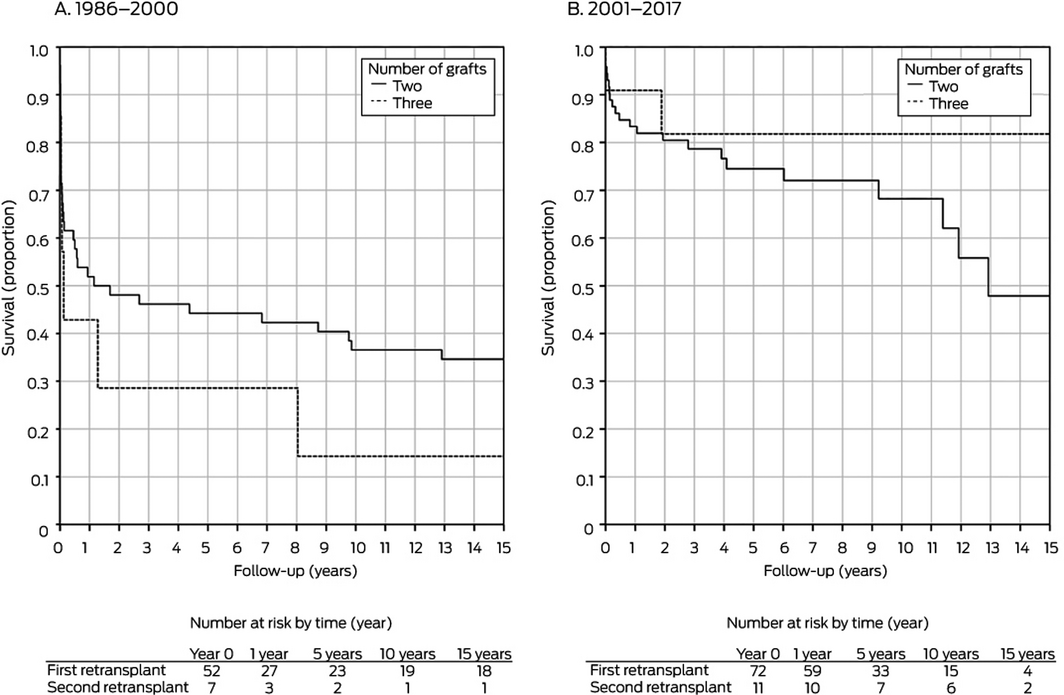
First v second retransplantation: 1986–2000, P = 0.18; 2000–2017, P = 0.32.
Box 5 – Graft survival following liver retransplantation in children, 1986–2017, by type of graft and transplantation period
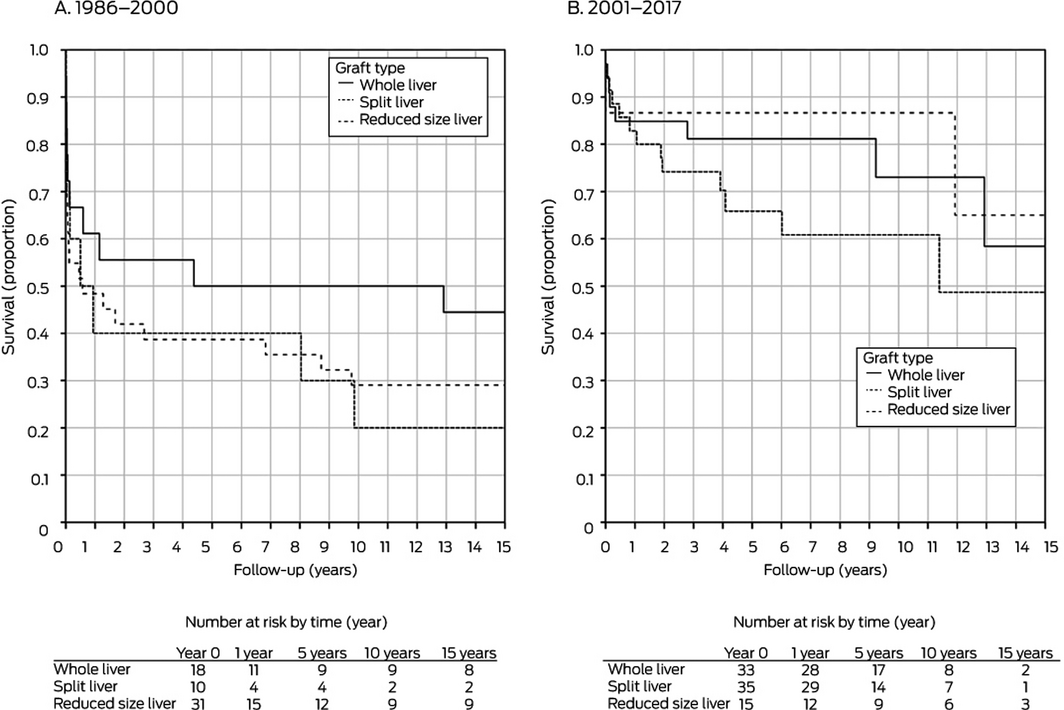
Graft type: 1986–2000, P = 0.59; 2000–2017, P = 0.29.
Box 6 – Causes of graft failure requiring liver retransplantation in children, 1986–2017
|
Cause of graft failure |
All |
1986–2000 |
2001–2017 |
||||||||||||
|
|
|||||||||||||||
|
All graft failures |
142 |
59 |
83 |
||||||||||||
|
Graft non‐function |
13 (9%) |
10 (17%) |
3 (4%) |
||||||||||||
|
Hepatic artery thrombosis or portal vein thrombosis |
55 (39%) |
31 (53%) |
24 (29%) |
||||||||||||
|
Biliary disease |
23 (16%) |
1 (2%) |
22 (27%) |
||||||||||||
|
Disease recurrence |
5 (4%) |
1 (2%) |
4 (5%) |
||||||||||||
|
Rejection (acute or chronic) |
40 (28%) |
16 (27%) |
24 (29%) |
||||||||||||
|
Other |
6 (4%) |
0 |
6 (7%) |
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 7 – Causes of graft failure requiring liver retransplantations in children, 1986–2017, by liver graft type
|
Cause of graft failure |
1986–2000 |
2001–2017 |
|||||||||||||
|
Split graft |
Reduced size graft |
Whole graft |
Split graft |
Reduced size graft |
Whole graft |
||||||||||
|
|
|||||||||||||||
|
All graft failures |
10 |
31 |
18 |
35 |
15 |
33 |
|||||||||
|
Graft non‐function |
1 (10%) |
7 (23%) |
2 (11%) |
7 (20%) |
3 (20%) |
1 (3%) |
|||||||||
|
Hepatic artery or portal vein thrombosis |
8 (80%) |
18 (58%) |
5 (28%) |
11 (31%) |
5 (33%) |
8 (24%) |
|||||||||
|
Biliary disease |
0 |
1 (3%) |
0 |
7 (20%) |
3 (20%) |
12 (36%) |
|||||||||
|
Disease recurrence |
0 |
0 |
1 (6%) |
1 (3%) |
0 |
3 (9%) |
|||||||||
|
Rejection (acute or chronic) |
1 (10%) |
5 (16%) |
10 (55%) |
5 (14%) |
3 (20%) |
8 (24%) |
|||||||||
|
Other |
0 |
0 |
0 |
4 (11%) |
1 (7%) |
1 (3%) |
|||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 9 – Graft and patient survival for children undergoing liver retransplantation, by country
|
|
Patients |
Patient survival |
Graft survival |
||||||||||||
|
1 year |
5 years |
10 years |
1 year |
5 years |
10 years |
||||||||||
|
|
|||||||||||||||
|
Australia and New Zealand, all retransplantations, 1986–2000 |
142 |
75% |
74% |
71% |
70% |
61% |
54% |
||||||||
|
Australia and New Zealand, all retransplantations, 2001–2017 |
83 |
89% |
87% |
87% |
84% |
75% |
70% |
||||||||
|
United States UNOS database, first retransplantation: 1989–200614 |
1274 |
NR |
NR |
NR |
60% |
50% |
46% |
||||||||
|
United States SPLIT database, all retransplantations, 1995–20043 |
242 |
67% |
59%* |
NR |
59% |
49%* |
NR |
||||||||
|
Atlanta: all retransplantations, 1997–200922 |
34 |
91% |
84%† |
NR |
87% |
74%† |
NR |
||||||||
|
|
|||||||||||||||
|
NR = not reported; SPLIT = Studies of Pediatric Liver Transplantation Registry Database; UNOS = United Network for Organ Sharing. * 4‐year survival. † 3‐year survival. |
|||||||||||||||
Received 16 March 2020, accepted 10 July 2020
- Angus W Jeffrey1
- Gary P Jeffrey1,2
- Michael Stormon3,4
- Gordon Thomas3,4
- Edward O'Loughlin3,4
- Albert Shun3,4
- Winita Hardikar5
- Robert Jones6,7
- John McCall8,9
- Helen Evans9
- Graham Starkey6,7
- Peter Hodgkinson10,11
- Looi C Ee12
- David Moore13
- Catherine Mews14
- Geoff W McCaughan15,16
- Peter W Angus6,7
- Alan J Wigg17
- Michael Crawford4,15
- Jonathan Fawcett10,11
- 1 Sir Charles Gairdner Hospital, Perth, WA
- 2 The University of Western Australia, Perth, WA
- 3 Australian National Liver Transplantation Service, Children's Hospital at Westmead, Sydney, NSW
- 4 The University of Sydney, Sydney, NSW
- 5 Royal Children's Hospital, Melbourne, VIC
- 6 Victorian Liver Transplant Unit, Austin Hospital, Melbourne, VIC
- 7 Victorian Liver Transplant Unit, Royal Children's Hospital, Melbourne, VIC
- 8 New Zealand Liver Transplant Unit, Auckland City Hospital, Auckland, New Zealand
- 9 Starship Children's Health, Auckland, New Zealand
- 10 Queensland Liver Transplantation Service, Princess Alexandra Hospital, Brisbane, QLD
- 11 The University of Queensland, Brisbane, QLD
- 12 Lady Cilento Children's Hospital, Brisbane, QLD
- 13 Women's and Children's Hospital, Adelaide, SA
- 14 Perth Children's Hospital, Perth, WA
- 15 Australian National Liver Transplantation Unit, Royal Prince Alfred Hospital, Sydney, NSW
- 16 Sydney Medical School, , the University of Sydney, Sydney, NSW
- 17 South Australian Liver Transplantation Service, Flinders Medical Centre, Adelaide, SA
No relevant disclosures.
- 1. Goss JA, Shackleton CR, McDiarmid SV, et al. Long‐term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411–420.
- 2. Sánchez‐Bueno F, Acosta F, Ramirez P, et al. Incidence and survival rate of hepatic retransplantation in a series of 300 orthotopic liver transplants. Transplant Proc 2000; 32: 2671–2672.
- 3. Ng V, Anand R, Martz K, Fecteau A. Liver retransplantation in children: a SPLIT database analysis of outcome and predictive factors for survival. Am J Transplant 2008; 8: 386–395.
- 4. Deshpande RR, Rela M, Girlanda R, et al. Long‐term outcome of liver retransplantation in children. Transplantation 2002; 74: 1124–1130.
- 5. Sieders E, Peeters PMJG, TenVergert EM, et al. Retransplantation of the liver in children. Transplantation 2001; 71: 90.
- 6. Newell KA, Millis JM, Bruce DS, et al. An analysis of hepatic retransplantation in children. Transplantation 1998; 65: 1172.
- 7. Ogura Y, Kaihara S, Haga H, et al. Outcomes for pediatric liver retransplantation from living donors. Transplantation 2003; 76: 943.
- 8. Kim WR, Lake JR, Smith JM, et al. Liver. Am J Transplant 2016; 16 (Suppl 2): 69–98.
- 9. Fink MA, Berry SR, Gow PJ, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol 2007; 22: 119–124.
- 10. Transplantation Society of Australia and New Zealand. Clinical guidelines for organ transplantation from deceased donors; version 1.3. May 2019. https://tsanz.com.au/guidelinesethics-documents/organallocationguidelines.htm (viewed Mar 2020).
- 11. Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor‐organ shortage. Lancet 1999; 354: 1636–1639.
- 12. Biggins SW. Futility and rationing in liver retransplantation: when and how can we say no? J Hepatol 2012; 56: 1404–1411.
- 13. Huesch MD. One and done? Equality of opportunity and repeated access to scarce, indivisible medical resources. BMC Med Ethics 2012; 13: 11.
- 14. Davis A, Rosenthal P, Glidden D. Pediatric liver retransplantation: outcomes and a prognostic scoring tool. Liver Transpl 2009; 15: 199–207.
- 15. Oswari H, Lynch SV, Fawcett J, et al. Outcomes of split versus reduced‐size grafts in pediatric liver transplantation. J Gastroenterol Hepatol 2005; 20: 1850–1854.
- 16. Vulchev A, Roberts JP, Stock PG. Ethical issues in split versus whole liver transplantation. Am J Transplant 2004; 4: 1737–1740.
- 17. Diamond IR, Fecteau A, Millis JM, et al. Impact of graft type on outcome in pediatric liver transplantation: a report from Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg 2007; 246: 301–310.
- 18. Australia and New Zealand Liver Transplant Registry. 30th annual ANZLITR report. Report on liver and intestinal transplantation activity to 31/12/2018. 2020. https://www3.anzltr.org/wp-content/uploads/Reports/30thReport.pdf (viewed Mar 2020).
- 19. Battula NR, Platto M, Anbarasan R, et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann Surg 2017; 265: 1009–1015.
- 20. McDiarmid SV, Anand R, Lindblad AS. Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end‐stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation 2002; 74: 173–181.
- 21. Jeffrey AW, Delriviere L, McCaughan G, et al. Excellent contemporary graft survival for adult liver retransplantation: an Australian and New Zealand registry analysis from 1986 to 2017. Transplant Direct 2019; 5: e427.
- 22. Heffron TG, Pillen T, Smallwood G, et al. Liver retransplantation in children: the Atlanta experience. Pediatr Transplant 2010; 14: 417–425.
- 23. McDiarmid SV, Merion RM, Dykstra DM, Harper AM. Selection of pediatric candidates under the PELD system. Liver Transpl 2004; 10 (Suppl 10): S23–S30.
- 24. Annunziato RA, Emre S, Shneider B, et al. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatr Transplant 2007; 11: 608–614.
- 25. Mitchell T, Gooding H, Mews C, et al. Transition to adult care for pediatric liver transplant recipients: the Western Australian experience. Pediatr Transplant 2017; 21: e12820.





Abstract
Objective: To assess long term graft and patient survival after donor liver retransplantation in children in Australia and New Zealand during 1986–2017; to determine the factors that influence survival.
Design: Retrospective cohort analysis (registry data).
Setting, participants: Australia and New Zealand Liver Transplant Registry data for all liver retransplantations in children (under 18 years of age), 1986–2017, in all four paediatric and six adult liver transplantation centres in the two countries.
Main outcome measures: Graft and patient survival at one, 5, 10 and 15 years.
Results: 142 liver retransplantations were undertaken in children (59 during 1986–2000, 83 during 2001–2017). Kaplan–Meier survival analysis indicated that survival was significantly greater during 2001–2017 than 1986–2000 (P < 0.001). During 2001–2017, graft survival one year after retransplantation was 84%, at 5 years 75%, at 10 years 70%, and at 15 years 54%; patient survival was 89% at one year, 87% at 5 years, 87% at 10 years, and 71% at 15 years. Median time between transplantations was 0.2 years (IQR, 0.03–1.4 years) during 1986–2000, and 1.8 years (IQR, 0.1–6.8 years) during 2001–2017 (P = 0.002). The proportion of graft failures that involved split grafts was larger during 2001–2017 (35 of 83, 42%) than 1986–2000 (10 of 59, 17%). Graft type, cause of graft failure, and number of transplants did not influence survival following retransplantation.
Conclusion: Survival for children following retransplantation is excellent. Graft survival is similar for split and whole grafts. Children on the liver waiting list requiring retransplantation should have the same access to donor grafts as children requiring a first transplant.