The known: Routine antenatal anti‐D prophylaxis reduces the risk of Rh(D) sensitisation of pregnant women. It is unclear whether the two‐dose regimen recommended in Australia is superior to the single dose regimen recommended in some countries.
The new: The proportion of women with detectable circulating anti‐D at delivery was greater for those who had received antenatal anti‐D prophylaxis as two separate doses than for women who received it as a single dose.
The implications: The current Australian recommendation of two‐dose antenatal anti‐D prophylaxis should be maintained, but strategies for increasing compliance are needed to better protect Rh(D)‐negative mothers.
Rhesus D (Rh(D)) isoimmunisation occurs when an Rh(D)‐negative mother forms Rh(D)‐immunoglobulin antibodies (anti‐D) in response to exposure to Rh(D)‐positive fetal red blood cells; these antibodies can cross the placenta during a subsequent pregnancy with an Rh(D)‐positive fetus and initiate immune‐mediated haemolysis. Maternal sensitisation most commonly follows exposure to fetal erythrocytes during labour, but may also occur antenatally as the result of antepartum haemorrhage, invasive fetal procedures, or asymptomatic feto‐maternal haemorrhage.1
During the 1960s and 1970s, administering prophylactic Rh(D)‐immunoglobulin (anti‐D) to Rh(D)‐negative mothers after delivery of a Rh(D)‐positive fetus and after potentially sensitising antenatal events was found to reduce the rate of sensitisation of Rh(D)‐negative mothers from 7–10% to 1–2% (Box 1).2,3,4 A large proportion of the instances of sensitisation despite prophylaxis are attributed to silent antenatal feto‐maternal haemorrhage events.1 Routine antenatal anti‐D prophylaxis (RAADP) was introduced to protect against such events and further reduced the rate of sensitisation to fewer than 0.4% of Rh(D)‐negative women who delivered Rh(D)‐positive fetuses.5,6,7,8,9,10
Anti‐D prophylaxis has dramatically reduced the incidence of Rh(D) disease in Australia since its introduction in the 1960s.11 RAADP is now recommended for all non‐sensitised Rh(D)‐negative pregnant women12,13 and for those not predicted to be carrying Rh(D)‐negative fetuses by non‐invasive cell‐free fetal DNA RHD genotyping.14 RAADP regimens vary between countries, ranging from two doses of anti‐D of at least 500 IU each at 28 and 34 weeks of pregnancy to a single 1500 IU dose at 28 weeks.15 The current Australian recommendation is two 625 IU doses at 28 and 34 weeks.12
In contrast to the two‐dose regimen, single dose regimens have been associated with lower rates of anti‐D detectability in maternal blood at the time of delivery (22% v 61%).16 This reduction in the proportion of women with detectable anti‐D is significant because anti‐D levels below the detection threshold are insufficient for protecting against sensitisation following 0.6 mL feto‐maternal haemorrhages, which occur in about 1% of women during the late third trimester.1 This suggests that the two‐dose regimen may be preferable for reducing the rate of antenatal sensitisation.
On the other hand, compliance with single dose regimens is significantly higher than for two‐dose courses in terms of receiving all doses during the recommended windows (78% v 67% in one study).17 Other advantages of a single dose regimen include its convenience and cost efficiency.18
The efficacy of the recommended Australian regimen for maintaining adequate circulating anti‐D levels until delivery has not been assessed; earlier studies have assessed the effectiveness of other anti‐D doses, ranging from 250 IU to 1500 IU.6,7,8,9,19 Further, data on compliance with the recommended regimen in a clinical setting have not been published. Given the potential benefits for compliance, convenience and cost of adopting a single dose RAADP regimen, our study compared the effectiveness of two‐dose RAADP (as recommended in Australia) with that of a one‐dose regimen (recommended in the United Kingdom). The primary outcome was detectable anti‐D levels in the maternal circulation at delivery; the secondary outcome was compliance with the regimen according to the recommended schedule.
Methods
The study was an open label, randomised controlled trial between May 2013 and November 2015. The participants were women who attended a Western Australian tertiary maternity hospital (King Edward Memorial Hospital for Women, Perth) for antenatal care and were at least 18 years of age, less than 30 weeks pregnant at the time of enrolment and yet to receive RAADP, were Rh(D)‐negative (negative antibody screen), and intended to deliver their baby at the hospital. Exclusion criteria were prior anti‐D sensitisation, any contraindication of anti‐D administration, and a history of isolated IgA deficiency. Eligible women were approached by a medical student (author JC) or research assistant (author BPV) not involved in their clinical care. Consenting participants were randomised 1:1 by sealed envelope in blocks of ten to receiving either standard RAADP (intramuscular injections of 625 IU anti‐D at both 28–30 and 34–36 weeks’ gestation) or one intramuscular injection of 1500 IU anti‐D (two 625 IU ampoules and one 250 IU ampoule combined as a single injection) at 28–30 weeks. Neither the participants nor the clinicians and researchers were blinded to treatment allocation. All other aspects of clinical care followed routine hospital guidelines; in particular, further doses of anti‐D were to be administered after potentially sensitising events, such as antepartum haemorrhage, external cephalic version, or invasive fetal diagnostic or therapeutic procedures.
Maternal blood was collected during labour or in the week preceding a planned caesarean delivery. Antibody screens were performed with the Ortho BioVue column agglutination technique (Ortho Clinical Diagnostics). Routine cord blood samples were used for neonatal blood typing. Postnatal anti‐D prophylaxis was administered to all mothers of Rh(D)‐positive neonates, with the dose determined by Kleihauer testing (minimum, 625 IU).
Compliance was defined as receiving each dose of a regimen during the recommended 2‐week intervals (28–30 weeks or 34–36 weeks). Women who did not receive any anti‐D, received a routine dose outside the recommended interval, or received the incorrect dose were deemed non‐compliant. Women in the two‐dose group whose pregnancy ended before the end of the 34–36 weeks’ gestation window were deemed compliant if the first dose was administered at 28–30 weeks.
Statistical analysis
Power calculations based on compliance and anti‐D detectability data from an earlier study17 indicated that a larger sample size was required for the secondary outcome (compliance) than for the primary outcome (detectability). A sample size of 284 was required to detect (with 90% power, α = 0.05) a reduction in non‐compliance from 33% to 16% with the single dose regimen, the reduction we defined as clinically meaningful. To allow for a 5% drop‐out rate, the target sample size was 300 women.
Categorical variables and proportions were compared in χ2 tests. A sensitivity analysis was restricted to data for women who were fully compliant with their allocated study regimen (received all doses of anti‐D within the recommended 2‐week time intervals and had an antibody screen at the time of delivery). Factors potentially associated with detectability of anti‐D at the time of delivery (prophylaxis regimen, maternal weight, interval between final dose and birth interval, gestation at birth) were analysed by univariate and multivariate logistic regression. Statistical analyses were performed with the graphical statistics package R 3.2.3 (R Foundation for Statistical Computing). Analyses were by intention to treat.
Ethics approval
Approval to conduct the trial was provided by the Human Research Ethics Committee of the Women and Newborn Health Service at the King Edward Memorial Hospital for Women (reference, 2013039EW). All participants provided written informed consent at the time of enrolment.
Trial registration
The trial was retrospectively registered with the Australian and New Zealand Clinical Trials Registry on 17 June 2013 (ACTRN12613000661774).
Results
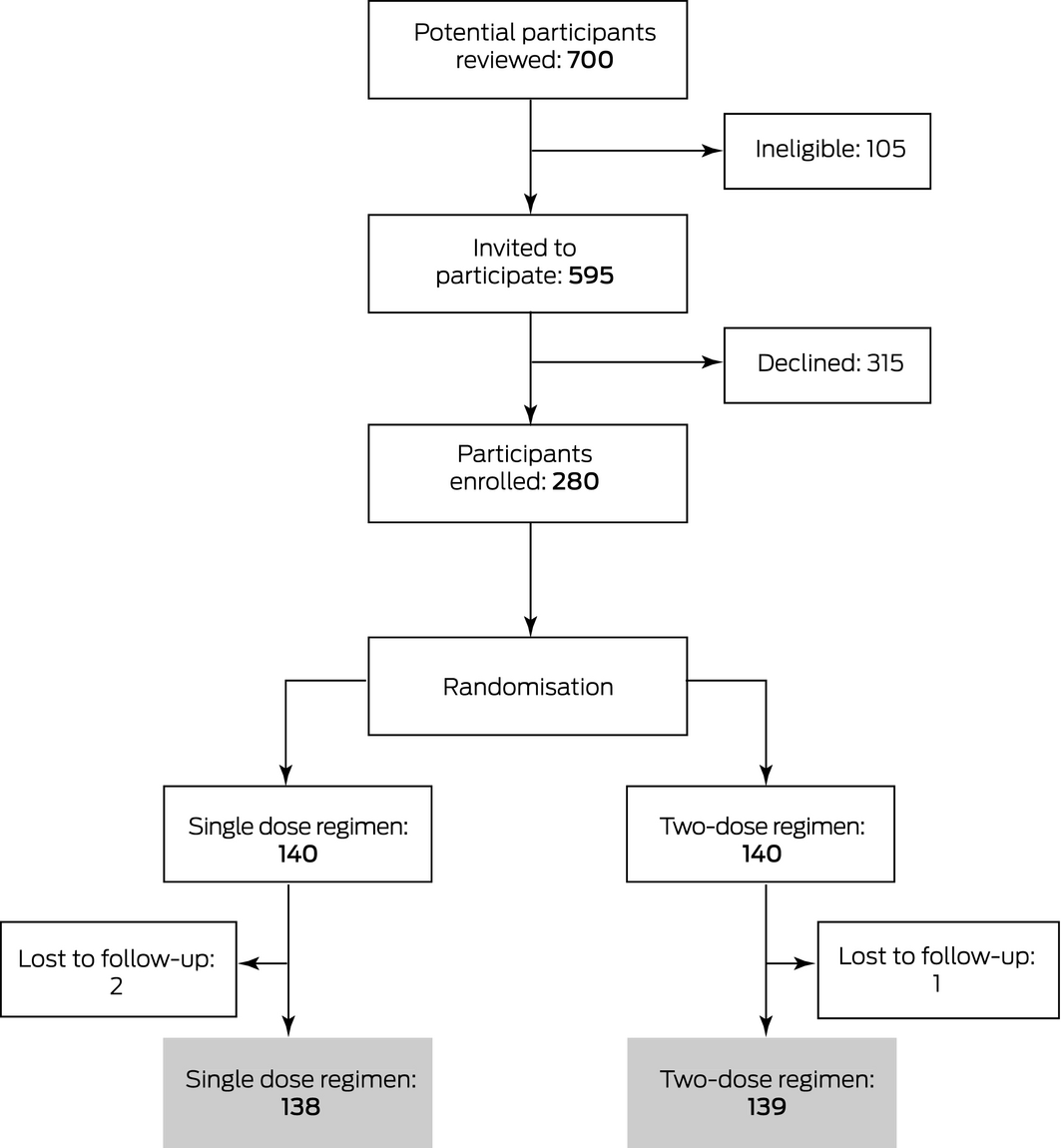
Recruitment ceased after 280 women had been recruited, as research assistance support for the trial had been exhausted. After three losses to follow‐up, 277 eligible participants were randomised to receive either single dose (138 women) or two‐dose RAADP (139 women) (Box 2). The demographic and birth characteristics of the two groups were similar (Box 3). None of these women required antenatal administration of additional anti‐D for potentially sensitising events after randomisation.
Anti‐D detectability at delivery
Antibody screens were performed at the time of their deliveries for 125 women in the single dose group (91%) and 129 in the two‐dose group (93%). Anti‐D was detectable in the maternal blood of more women in the two‐dose group than in the single dose group (111 of 129 [86%] v 70 of 125 [56%]; P < 0.001; odds ratio [OR], 4.91; 95% confidence interval [CI], 2.67–9.02; P < 0.001). In univariate analyses, increasing maternal weight (OR [per kg], 0.84; 95% CI, 0.76–0.93; P < 0.001) and interval between final dose and birth (OR [per day], 0.96; 95% CI, 0.95–0.98; P < 0.001) were associated with reduced detectability. After adjusting for these two factors, the association between two‐dose administration and detectability was not significant (adjusted OR, 1.55; 95% CI, 0.62–3.87; P = 0.35) (Box 4).
Compliance with RAADP regimen
Compliance with the RAADP regimen was low in both groups: 52 women (38%) in the single dose group and 69 (50%) in the two‐dose group did not receive the appropriate doses of anti‐D at the appropriate time points. The reasons for non‐compliance were different in the two groups: receiving an incorrect dose was more frequent in the single dose group (9% v 1%), while missing a dose was more common in the two‐dose group (10% v 4%), as were late doses (17% v 6%) (Box 5).
Safety
There were no major adverse events related to the trial. The greater injection volume (> 5 mL) for the single dose group initially made it more painful than for the standard regimen; the problem was alleviated by using a more concentrated product, delivering the same dose in a smaller volume (2 mL). Twelve women in the single dose group (9%) received only 625 IU anti‐D at 28–30 weeks; they were therefore given a second dose at 34–36 weeks, consistent with standard practice, to avoid potential late antenatal sensitisation.
Sensitivity analysis
A sensitivity analysis restricted to data for fully compliant participants assessed the effect of non‐compliance on the primary outcome. In this analysis, detection rates of anti‐D at delivery were 88% (57 of 65 screened women) in the two‐dose group and 56% (42 of 75 screened women) for the single dose group; that is, the rates were similar to those in the full analysis.
Discussion
Circulating anti‐D levels were too low for detection at delivery in significantly more women who received RAADP as a single dose rather than in women who received the standard two doses. Undetectably low levels leave women vulnerable to sensitisation in the event of asymptomatic feto‐maternal haemorrhages of 0.6 mL, which occur in 1% of pregnant women during their third trimester. Although the two‐dose RAADP regimen, standard in Australia, thus seems superior to a single dose approach, the absolute risk of sensitisation is likely to be small. The number needed to treat with two‐dose RAADP to prevent one case of undetectable anti‐D at birth is 3.1 based on the observed absolute risk reduction of 32%. However, only 1% of women with undetectable anti‐D levels at delivery are likely to be sensitised, so that one case of sensitisation could be prevented for about every 300 women by using two‐dose rather than single dose RAADP.
A longer time interval between women receiving their final dose of antenatal anti‐D and delivery was associated with increased risk of anti‐D not being detectable at delivery. It would be expected that the 1500 IU dose of the single dose regimen would produce a higher peak anti‐D level in the maternal circulation than the standard 625 IU dose, but its level may decline too rapidly to maintain adequate protection. The second dose of the two‐dose regimen is required to boost the circulating level of anti‐D, providing protection until term. The larger proportion of women with detectable anti‐D at delivery in the two‐dose group is probably related more to the shorter interval between administration of the final dose and delivery than to the regimen itself. A single 625 IU dose at 34 weeks may be as effective as two doses for providing detectable anti‐D levels at term, but this approach would leave women vulnerable to sensitisation by asymptomatic feto‐maternal haemorrhage events before 34 weeks.
Greater maternal weight was associated with a higher risk of anti‐D not being detectable at delivery, and the median weight of the women in the single dose group was greater than that for the two‐dose group. Despite the increased volume of distribution of administered anti‐D in heavier women and the consequently lower circulating levels achieved with equivalent doses, protection against sensitisation depends on the total amount of anti‐D, not its blood concentration; heavier women should not be at greater risk of sensitisation, as the concentration of fetal red blood cells in maternal blood will also be reduced. This interpretation is supported by the results of a case–control study (42 cases, 339 controls) that found no association between weight and sensitisation.20
Compliance with the allocated RAADP regimen was relatively low in both groups. The higher level among women in the single dose group (similar to the difference in an earlier study17) was not significantly different from that of the two‐dose group and was not associated with an improved rate of detectable anti‐D at delivery. As this was a pragmatic trial, clinicians were responsible for administering RAADP. It was anticipated that the Hawthorne effect (behavioural change caused by unconcealed observation) might improve clinician awareness and compliance with the treatment schedule, but compliance was poor in both groups, indicating that systematic improvements are required to ensure that RAADP is administered as recommended. A sensitivity analysis found that, even with optimal compliance, the anti‐D detectability rate at delivery was lower with the single dose regimen. Further, administering a second dose to the 12 women in the single dose group who had received only 625 IU at 28 weeks and the late administration of the 1500 IU dose to eight women would each be expected to increase the proportion of women in the single dose group with detectable anti‐D levels. Improving compliance with the single dose regimen would therefore not optimise protection of women against Rh(D) sensitisation; high anti‐D levels at delivery are more reliably provided by the two‐dose regimen if compliance is high.
Limitations
This study is limited by the relatively high incidence of protocol violations regarding anti‐D administration and the performance of antibody screens at the time of delivery. However, a sensitivity analysis suggests that this did not significantly influence the primary outcome. Compliance may vary according to the model of antenatal care employed, which may limit the applicability of our results to other care settings. Further, a considerable proportion of participants (7%) were not screened for antibody at the time of delivery, but the proportion was similar in the two groups.
Conclusion
Our trial provides indirect evidence for greater protection against Rh(D) sensitisation by the RAADP regimen recommended by Australian guidelines than by the single dose regimen used in some other countries. Systematic improvements that facilitate improved compliance with the current recommendations are needed to minimise the risk of isoimmunisation in Rh(D)‐negative women.
Box 1 – Timeline of Rh(D) immunoglobulin (anti‐D) prophylaxis for pregnant women in Australia
|
|
Before 1970 |
From 1970s |
From 2003 |
||||||||||||
|
|
|||||||||||||||
|
Anti‐D prophylaxis |
None |
Postnatal only |
Ante‐ and postnatal |
||||||||||||
|
Risk of sensitisation per birth |
10%2 |
1%2 |
|||||||||||||
|
Estimated number of Australian cases per year* |
5250 |
420 |
84 |
||||||||||||
|
|
|||||||||||||||
|
Estimated number of cases is based on 350 000 births per year and prevalence among pregnant women of Rh(D)‐negativity of 15%. Exact numbers are not available, as this information is not routinely collected. |
|||||||||||||||
Box 2 – CONSORT diagram of the selection of participants for our randomised controlled trial of Rh(D) immunoglobulin (anti‐D) prophylaxis
Box 3 – Demographic and birth characteristics of the participants, by allocated Rh(D) immunoglobulin (anti‐D) prophylaxis regimen
|
|
Anti‐D prophylaxis regimen |
||||||||||||||
|
Single dose |
Two‐dose |
||||||||||||||
|
|
|||||||||||||||
|
Number of women |
138 |
139 |
|||||||||||||
|
Age (years), mean (SD) |
30.9 (5.0) |
31.2 (5.0) |
|||||||||||||
|
Body mass index (kg/m2), median (IQR) |
26.2 (23.1–34.1) |
24.3 (21.8–29.4) |
|||||||||||||
|
Weight (kg), median (IQR) |
70.0 (60.4–88.8) |
66.0 (60.0–81.9) |
|||||||||||||
|
Multiple pregnancy |
3 (2%) |
4 (3%) |
|||||||||||||
|
Invasive fetal procedure |
4 (3%) |
4 (3%) |
|||||||||||||
|
Bleeding before 20th week of pregnancy |
7 (5%) |
15 (11%) |
|||||||||||||
|
Antepartum haemorrhage |
6 (4%) |
6 (4%) |
|||||||||||||
|
Spontaneous vaginal birth |
80 (58%) |
93 (67%) |
|||||||||||||
|
Instrumental birth |
15 (11%) |
17 (12%) |
|||||||||||||
|
Caesarean delivery |
37 (27%) |
43 (31%) |
|||||||||||||
|
Gestation at birth (weeks), median (IQR) |
39.0 (37.9–40.0) |
39.4 (37.9–40.6) |
|||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 4 – Detectability of Rh(D) immunoglobulin (anti‐D) in maternal blood at the time of delivery: multivariate analysis
|
Variable |
Univariate analysis |
Multivariate analysis |
|||||||||||||
|
Odds ratio (95% CI) |
P |
Adjusted odds ratio* (95% CI) |
P |
||||||||||||
|
|
|||||||||||||||
|
Two‐dose regimen |
4.91 (2.67–9.02) |
< 0.001 |
1.55 (0.62–3.87) |
0.35 |
|||||||||||
|
Maternal weight (per kg) |
0.84 (0.76–0.93) |
< 0.001 |
0.80 (0.71–0.92) |
0.001 |
|||||||||||
|
Interval: final dose to birth (per day) |
0.96 (0.95–0.98) |
< 0.001 |
0.97 (0.95–0.98) |
< 0.001 |
|||||||||||
|
Gestation at birth (per day) |
0.99 (0.97–1.01) |
0.20 |
— |
— |
|||||||||||
|
|
|||||||||||||||
|
Adjusted for regimen, maternal weight and final dose to birth interval. |
|||||||||||||||
Box 5 – Non‐compliance with Rh(D) immunoglobulin (anti‐D) prophylaxis regimen, by allocated regimen
|
|
Anti‐D prophylaxis regimen |
P |
|||||||||||||
|
Single dose |
Two doses |
||||||||||||||
|
|
|||||||||||||||
|
Number of women |
138 |
139 |
|
||||||||||||
|
Non‐compliant |
52 (39%) |
69 (50%) |
0.06 |
||||||||||||
|
Dose omitted |
5 (4%) |
14 (10%) |
0.037 |
||||||||||||
|
Dose early |
27 (20%) |
31 (22%) |
0.62 |
||||||||||||
|
Dose late |
8 (6%) |
23 (17%) |
0.005 |
||||||||||||
|
Dose incorrect |
12 (9%) |
1 (1%) |
0.002 |
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 28 November 2018, accepted 8 April 2019
- Scott W White1,2
- Janice C Cheng3
- Blagica Penova‐Veselinovic1
- Carol Wang4
- Melanie White4
- Bernie Ingleby2
- Christine Arnold2
- Craig E Pennell4,5
- 1 University of Western Australia, Perth, WA
- 2 King Edward Memorial Hospital for Women, Perth, WA
- 3 Royal Perth Hospital, Perth, WA
- 4 University of Newcastle, Newcastle, NSW
- 5 Hunter Medical Research Institute, Newcastle, NSW
This investigation was funded in part by a grant to Scott White from the Women and Infants Research Foundation (Perth).
No relevant disclosures.
- 1. Bowman JM, Pollock JM. Failures of intravenous Rh immune globulin prophylaxis: an analysis of the reasons for such failures. Transfus Med Rev 1987; 1: 101–112.
- 2. Woodrow JC, Donohoe WT. Rh‐immunization by pregnancy: results of a survey and their relevance to prophylactic therapy. Br Med J 1968; 4: 139–144.
- 3. Bowman JM, Chown B, Lewis M, Pollock JM. Rh isoimmunization during pregnancy: antenatal prophylaxis. Can Med Assoc J 1978; 118: 623–627.
- 4. Crowther C, Middleton P. Anti‐D administration after childbirth for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev 2000; CD000021.
- 5. MacKenzie IZ, Bichler J, Mason GC, et al. Efficacy and safety of a new, chromatographically purified rhesus (D) immunoglobulin. Eur J Obstet Gynecol Reprod Biol 2004; 117: 154–161.
- 6. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynaecol 1999; 106: 492–497.
- 7. Bowman JM, Pollock JM. Antenatal prophylaxis of Rh isoimmunization: 28‐weeks’‐gestation service program. Can Med Assoc J 1978; 118: 627–30.
- 8. Tovey LA, Townley A, Stevenson BJ, Taverner J. The Yorkshire antenatal anti‐D immunoglobulin trial in primigravidae. Lancet 1983; 2: 244–246.
- 9. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ 1997; 315: 1588.
- 10. Trolle B. Prenatal Rh‐immune prophylaxis with 300 micrograms immune globulin anti‐D in the 28th week of pregnancy. Acta Obstet Gynecol Scand 1989; 68: 45–47.
- 11. Thyer J, Wong J, Thomson A, et al. Fifty years of RhD immunoglobulin (anti‐D) therapy in Australia: celebrating a public health success story. Med J Aust 2018; 209: 336–339. https://www.mja.com.au/journal/2018/209/8/fifty-years-rhd-immunoglobulin-anti-d-therapy-australia-celebrating-public.
- 12. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Guidelines for the use of Rh(D) immunoglobulin (Anti‐D) in obstetrics in Australia. Reviewed Nov 2015. https://www.ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Guidelines-for-the-use-of-Rh(D)-Immunoglobulin-(Anti-D)-(C-Obs-6)-Review-November-2015.pdf?ext=.pdf (viewed May 2019).
- 13. Qureshi H, Massey E, Kirwan D, et al. BCSH guideline for the use of anti‐D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med 2014; 24: 8–20.
- 14. Haimila K, Sulin K, Kuosmanen M, et al. Targeted antenatal anti‐D prophylaxis program for RhD‐negative pregnant women: outcome of the first two years of a national program in Finland. Acta Obstet Gynecol Scand 2017; 96: 1228–1233.
- 15. Sperling JD, Dahlke JD, Sutton D, et al. Prevention of RhD alloimmunization: a comparison of four national guidelines. Am J Perinatol 2018; 35: 110–119.
- 16. Davies J, Chant R, Simpson S, Powell R. Routine antenatal anti‐D prophylaxis: is the protection adequate? Transfus Med 2011; 21: 421–426.
- 17. MacKenzie IZ, Dutton S, Roseman F. Evidence to support the single‐dose over the two‐dose protocol for routine antenatal anti‐D Rhesus prophylaxis: a prospective observational study. Eur J Obstet Gynecol Reprod Biol 2011; 158: 42–46.
- 18. Pilgrim H, Lloyd‐Jones M, Rees A. Routine antenatal anti‐D prophylaxis for RhD‐negative women: a systematic review and economic evaluation. Health Technol Assess 2009; 13 (no. 10).
- 19. Lee D, Rawlinson VI. Multicentre trial of antepartum low‐dose anti‐D immunoglobulin. Tranfus Med 1995; 5: 15–19.
- 20. Koelewijn JM, de Haas M, Vrijkotte TG, et al. Risk factors for RhD immunisation despite antenatal and postnatal anti‐D prophylaxis. BJOG 2009; 116: 1307–1314.





Abstract
Objective: To compare rates of detectability of circulating Rh(D)‐immunoglobulin (anti‐D) at delivery with single and two‐dose antenatal anti‐D prophylaxis (RAADP) regimens; to compare compliance with the two regimens.
Design: Open label, randomised controlled trial between May 2013 and November 2015.
Setting, participants: 277 women who attended a tertiary obstetric referral hospital in Perth for antenatal care and were at least 18 years of age, less than 30 weeks pregnant and yet to receive RAADP, Rh(D)‐negative (negative antibody screen), and who intended to deliver their baby at the hospital. Exclusion criteria were prior anti‐D sensitisation, any contraindication of anti‐D administration, and a history of isolated IgA deficiency.
Interventions: One 1500 IU anti‐D dose at 28 weeks of pregnancy (single dose regimen); two doses of 625 IU each at 28 and 34 weeks of pregnancy (two‐dose regimen).
Main outcome measures: The primary outcome was the proportion of women with detectable anti‐D levels at delivery; the secondary outcome was compliance with the allocated RAADP regimen.
Results: Circulating anti‐D was detectable at delivery in a greater proportion of women in the two‐dose group (111 of 129, 86%) than in the single dose group (70 of 125, 56%; P < 0.001). Compliance was not significantly different between the single dose (86 of 138, 61%) and two‐dose groups (70 of 139, 50%; P = 0.06).
Conclusions: The two‐dose RAADP schedule currently recommended in Australia provides better protection against Rh(D) sensitisation than a one‐dose regimen.
Trial registration: Australian and New Zealand Clinical Trials Registry (ACTRN12613000661774).