The known: Recommendations regarding directly referring patients from primary care for assessment for obstructive sleep apnoea (OSA) according to screening questionnaire responses rest not on firm evidence but on expert opinion.
The new: The combination of screening questionnaires and the Epworth sleepiness scale (ESS) is useful for selecting patients for sleep testing, but misses more than half of those with clinically relevant OSA. The sensitivity and specificity of screening could be adjusted to the primary care needs of particular health care systems with our STOP‐Bang/ESS‐based decision support tool.
The implications: Questionnaire‐based screening cannot currently be employed in primary care to rule out OSA.
Obstructive sleep apnoea (OSA) affects 9–38% of adults,1 is associated with high morbidity and mortality, and its health‐related and other costs are high.2,3 Although early diagnosis and treatment can reduce the burden for the patient, OSA is underdiagnosed4 because there are no recommendations about screening and diagnostic polysomnography is expensive.5 Streamlining the diagnosis and management of OSA in primary care is therefore of great importance.
Until recently, OSA was predominantly managed by sleep specialists, although there are markedly different models of care.6 Some primary care centres assess patients for OSA with screening questionnaires, but there are no formal standards.3 Some use the Epworth sleepiness scale (ESS),7 which measures excessive daytime sleepiness, while others screen with instruments such as the Berlin (BQ),8 STOP‐Bang9 and OSA‐50 questionnaires.10 In clinical settings, a questionnaire‐based assessment should ideally produce as few false positive and false negative results as possible, to minimise both demands on the health care system and unnecessary anxiety for patients, and to ensure that beneficial treatment will be provided to people who need it.
Some initiatives for streamlining referral for diagnostic sleep studies and treatment have been promising. In Spain, for example, diagnosing and managing patients with moderate to severe, highly symptomatic OSA (ESS score of 12 or more) in primary care was found to be non‐inferior to specialist management.11 In Australia, the universal health care funder (Medicare) has recently accepted changes to its schedule of payable items that allow primary care clinicians to directly refer patients for sleep studies if they have a positive BQ result in at least two categories, an OSA‐50 score of at least 5, or a STOP‐Bang score of at least 4, if they also have an ESS score of 8 or more.12
Although OSA screening questionnaires are increasingly important for decision making in primary care, their performance in this setting has not been assessed.3 We therefore investigated the utility of the BQ, STOP‐Bang, and OSA‐50 questionnaires in primary care, alone and in combination with the ESS, with the aim of determining whether they could be used to streamline the management of OSA in primary care.
Methods
Study design
Our study was performed within the Tasmanian Longitudinal Health Study (TAHS), 6th Decade Follow‐up (TAHS‐6). The inception and serial follow‐ups of the TAHS have been reported previously.13 In brief, 8583 Tasmanian school children born in 1961 were recruited in 1968 and followed up in 1974, 1979, 1991, 2002, 2010 and 2012. Valid contact details were available in 2012 for 6128 of the original participants (71%), and they were invited to participate in TAHS‐6, which was completed in 2016.
Participants and selection process
Of 3609 participants in TAHS‐6, 2659 (73.7%) visited partner respiratory laboratories and completed questionnaires. A random sample of 772 participants were then invited to undergo sleep studies, irrespective of their questionnaire responses, of whom 424 (54.9%) participated in home‐based type 4 sleep studies (that is, studies that assess only one or two sleep parameters) using portable ApneaLink devices (ResMed).
Test methods
The ApneaLink device has channels for heart rate, oxygen haemoglobin saturation (measured by pulse oximetry), and nasal airflow pressure (recorded through a nasal cannula connected to a pressure transducer). Signals were automatically analysed with proprietary software (ApneaLink 9.2.0) that calculated the apnoea–hypopnea index (AHI; based on airflow limitation events; ie, airflow 50% lower than baseline) and oxygen desaturation index (ODI; based on oxygen desaturation events; ie, 3% lower than baseline). We used the ODI to define the degree of OSA, as oxygen desaturation has been linked with the adverse consequences of OSA;14,15,16 further, ODI performs as well in this respect as the derived AHI, but pulse oximetry yields a more robust signal than nasal airflow transduction, with fewer test failures.17
Participants completed BQ, STOP‐Bang, OSA‐50, and ESS questionnaires (online Supporting Information, supplementary methods). Height, weight, and waist, hip and neck circumferences were measured. We regarded troublesome snoring, witnessed apnoeas, and excessive sleepiness or fatigue, as reported in the questionnaires, as likely triggers for a patient to visit a primary care physician and for the latter to initiate assessment of OSA (Supporting Information, table 1).
Definitions
The ESS is an eight‐item questionnaire that assesses subjective sleepiness; cut‐off scores of 8 and 11 (out of 24) have each been proposed as indicating excessive day time sleepiness;7,18 we applied an ESS cut‐off score of 8.
High risk of OSA was defined by standard cut‐off scores for the screening questionnaires: at least two positive categories of three for the BQ,8 a score of 3 or more out of 8 for STOP‐Bang,9 and a score of 5 or more out of 10 for OSA‐50.10
Participants with 5–14 ODI events/hour were defined as having mild OSA, those with 15 or more events/hour as having moderate to severe OSA.19
Mild OSA is common in the general population,1 but its significance is uncertain, so it is usually not treated unless symptomatic (eg, accompanied by excessive day time sleepiness); moderate to severe OSA, however, is often treated without regard to symptom severity.3 We therefore deemed people who had either moderate to severe OSA, or mild OSA together with excessive day time sleepiness, as having clinically relevant OSA.
An affirmative response to at least one of the ten questions on troublesome snoring (three questions), witnessed apnoea (three questions), and sleepiness or fatigue/tiredness (four questions; online Supporting Information, table 1) was defined as indicating a trigger symptom.
Sensitivity and specificity were deemed high if they exceeded 90%, fair if 71–90%, and low if 70% or less.
Data analysis
Data were included in our analysis if at least 4 hours of oxygen desaturation data were available for a participant with at least one trigger symptom. Receiver operator characteristic (ROC) curves20 were generated and areas under ROC curves calculated for each screening questionnaire. Validity parameters were estimated (with 95% confidence intervals [CIs]). The analyses were repeated for each screening questionnaire and for different STOP‐Bang cut‐off scores combined with ESS scores of 8 or more. The justification for using ESS scores in both the reference and index tests is discussed in the online Supporting Information.
We also conducted sensitivity analyses of data for participants who were married or in de facto relationships (as people with partners are more likely to report nocturnal events), and by using AHI instead of ODI to define OSA. A sub‐analysis of patients with moderate to severe OSA was also undertaken. All analyses were conducted in Stata/SE 14.1 (StataCorp).
Ethics approval
This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the Human Research Ethics Committee of the University of Tasmania (approval number, H0012710).
Results
For 286 participants, at least 4 hours of sleep study recordings were available and at least one trigger symptom was identified (Box 1). Their mean age was 52.9 years (standard deviation [SD], 0.9 year), their mean body mass index (BMI) was 29.3 kg/m2 (SD, 5.3 kg/m2), and 152 were men (53%) (Box 2). A total of 232 participants (81%) had some degree of OSA (mild, 141 [49%], moderate to severe, 91 [32%]); 140 had clinically relevant OSA (49%) (Box 1).
The characteristics (sex, BMI, neck circumference, presence of hypertension, high risk of OSA according to BQ and STOP‐Bang) of the 772 people randomly invited to participate in the sleep studies were similar to those of the people who were not invited (data not shown). Mean ESS scores were higher for people who underwent sleep studies (5.8; standard deviation [SD], 4.0) than for those who did not (5.1; SD, 3.8; P = 0.020), and were also higher for sleep study participants for whom at least 4 hours’ oximetry data were available (5.9; SD, 4.1) than for those for whom it was not (5.1; SD, 3.8; P = 0.010) (Supporting Information, table 2).
Diagnostic utility of standard cut‐off scores of BQ, STOP‐Bang, and OSA‐50
The sensitivity of the STOP‐Bang (81%) and OSA‐50 (86%) was fair, but their specificity was low (36% and 21% respectively; Box 3, Box 4, Supporting Information, table 3); the sensitivity of the BQ was lower (65%) and its specificity higher, although still low (59%). The specificity of combinations of standard questionnaire cut‐off scores with an ESS cut‐off score of 8 was high (92–95%; Box 4, Box 5) but their sensitivity was low (36–51%). The ability of the questionnaires to detect moderate to severe OSA was similar, but sensitivity and specificity were lower when their results were combined with an ESS cut‐off score of 8 (Supporting Information, table 4, figures 1 and 2).
Diagnostic utility of different STOP‐Bang cut‐off scores
As different STOP‐Bang cut‐off scores could be applied in different settings,21 we assessed the diagnostic utility of different cut‐off scores for detecting clinically relevant OSA (Supporting Information, table 5). Specificity was greater (and sensitivity lower) with higher cut‐off scores; accuracy (true positive and negative results as a proportion of all results) was greatest at the standard cut‐off score of 3, but still poor (63%). When combined with an ESS cut‐off score of 8, specificity was high for all STOP‐Bang cut‐offs above 2 (score of 5, 99%; of 6 or 7, 100%; Supporting Information, table 6).
The diagnostic utility of screening questionnaires was similar when only data for people who were married or in de facto relationships were analysed, and when AHI was used instead of ODI to define OSA (data not shown). Two‐step screening — applying the highly sensitive OSA‐50 (OSA ruled out by negative result) and then assessing people with positive OSA‐50 results with the more specific BQ — did not improve the sensitivity or specificity of the overall screening process (data not shown).
Screening questionnaires in primary care
A decision support tool based on our findings can be adapted for decision making in primary care settings (Box 6). The proportion of people with clinically relevant OSA that would be missed is defined by 1 – sensitivity; the proportion of healthy people who would be referred for further assessment is defined by 1 – specificity. This information can be used to generate assessment and referral models that suit the requirements of each primary care practice or health care system.
Discussion
For a sample of middle‐aged people with OSA‐related symptoms likely to present to primary care clinics, the sensitivity and specificity of three screening questionnaires were not useful for detecting clinically significant OSA, but high specificity could be achieved, at the cost of reduced sensitivity, by combining the scores on any of these instruments with a high ESS score criterion. As expected, raising the STOP‐Bang cut‐off score increased the specificity and reduced the sensitivity of the instrument, both alone and when combined with high ESS scores.
Implications of our findings
If used to rule out OSA in primary care settings, the three screening questionnaires would exclude 14–35% of people with clinically relevant OSA. Although adding the second criterion of an ESS score of 8 or more for ruling in OSA increased the specificity of screening from 21–59% to 92–95%, this combination missed 49–64% of participants with clinically relevant OSA (Box 4). On the other hand, different STOP‐Bang cut‐off scores could be applied to suit the specificity and sensitivity requirements of particular primary care settings or health care systems when assessing patients for OSA; that is, according to whether reducing the burdening on diagnostic facilities or detecting as much OSA risk as possible is more important.
Despite recent attempts to streamline OSA‐related decision making at the primary care level, including in Spain11 and Australia,12 evidence supporting these approaches is scarce. The decision by the Australian Department of Health to accept a positive BQ, STOP‐Bang (with a cut‐off score of 4) or OSA‐50 result, coupled with an ESS score of 8 or more, as criteria for direct referral for sleep studies by primary care physicians12 is the first Australian policy initiative with this aim. But it remains unclear how the large proportion of people with clinically relevant OSA who do not meet these criteria (more than half) should be managed.
Our decision support tool (Box 6) may help standardise the assessment and referral process, although it requires further validation in other populations and by polysomnography. In patients with an ESS score of 8 or more, reducing the STOP‐Bang cut‐off score for referral for sleep studies from 4 to 3 would enhance detection of patients with significant OSA (from 30% to 50%) without markedly increasing the number of false positives (from 6% to 8%). A universal decision model is not possible because of differences in the resources available to different health care systems, but our support tool could be adapted to the requirements of particular systems and inform decisions about allocating the limited resources of sleep clinics and laboratories.
Finally, the poor performance of screening questionnaires, alone and when combined with ESS scores, in correctly identifying people with OSA indicates a need for alternative screening methods, either improved versions of currently available screening questionnaires or a new questionnaire, or a two‐step screening process.
Strengths and limitations of our study
Our study is the first in Australia to regard clinically relevant OSA as the condition that needs managing at the primary care level, given the uncertain clinical relevance of the more prevalent mild OSA.1 Daytime sleepiness (as measured with the ESS) was used to both define clinically relevant OSA and to help refine the results of screening questionnaires, reflecting clinical practice.
Our study included a population sample from an age group that could benefit from the early diagnosis and treatment of OSA. However, the low participation rate in TAHS‐6 may have led to selection bias. We used a level 4 sleep study as the reference standard for defining clinically relevant OSA.22 Portable devices are of varying diagnostic utility,3,22,23 but the performance of ApneaLink is comparable with assessment of AHI in a sleep laboratory, particularly in people with moderate to severe OSA.23 The device cannot distinguish obstructive and central respiratory events, but this was unlikely to have markedly affected our results, as the population prevalence of central sleep apnoea is negligible in women and about 0.4% in men.14,24 Further, we probably underestimated the prevalence of clinically relevant OSA by not scoring electroencephalography‐based arousals in the absence of desaturation in the auto‐analysed ODI3% and not limiting scored events to periods of sleep, both of which are standard for manually scored type 2 sleep studies.25
On the other hand, people with ESS scores of 8 or more, particularly those with mild OSA, could be sleepy for reasons other than OSA, which could partly explain the relatively high prevalence of clinically relevant OSA we found. As the screening questionnaires have been validated in populations not pre‐selected according to their symptoms, our recruiting a sample of people with the OSA‐related symptoms covered by the questionnaires would have led to a large proportion of false positives. However, questionnaires are more likely to be used in clinical practice after a patient has reported symptoms. As both age and hypertension are assessed by OSA screening questionnaires, the diagnostic utility of these tools is likely to be different in younger patients or a population with a broader age distribution.
Conclusions
The BQ, STOP‐Bang and OSA‐50 questionnaires, together with an ESS score of 8 or more, can be used to rule in clinically relevant OSA, but these criteria exclude more than half the patients with clinically relevant OSA if used to rule out OSA. Further work is required to determine how these patients can be efficiently identified so that they can receive appropriate treatment. Our STOP‐Bang/ESS‐based decision support tool may assist primary care physicians make objective and uniform decisions regarding OSA assessment and referral.
Box 1 – The selection of participants with at least four hours of sleep study data and at least one trigger symptom
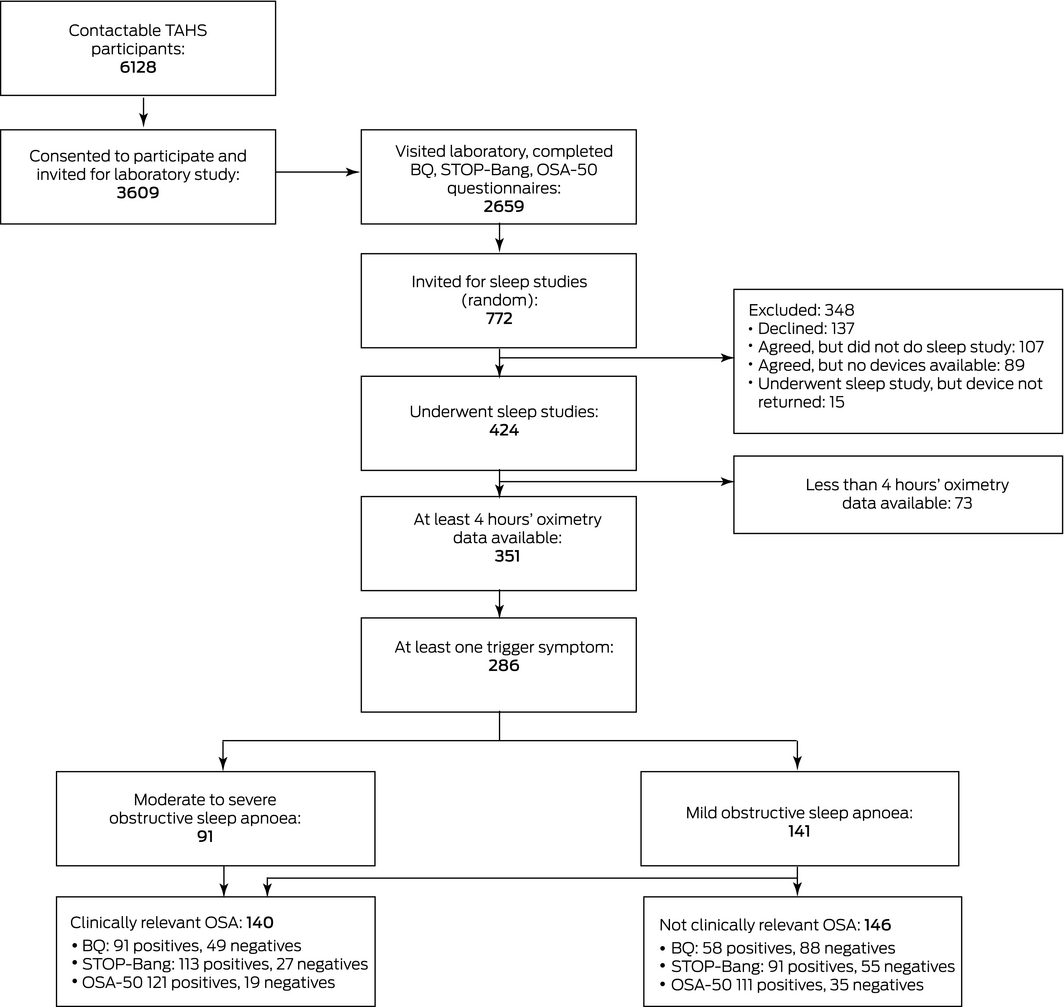
BQ = Berlin questionnaire; TAHS = Tasmanian Longitudinal Health Study. ◆
Box 2 – Characteristics of the 286 participants for whom at least 4 hours’ sleep study recordings were available and who had at least one trigger symptom
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Age (years), mean (SD) |
52.9 (0.9) |
||||||||||||||
|
Sex (men) |
152 (53%) |
||||||||||||||
|
Body mass index (kg/m2), mean (SD) |
29.3 (5.3) |
||||||||||||||
|
Neck circumference (cm), mean (SD) |
38.0 (3.8) |
||||||||||||||
|
Berlin score ≥ 2 |
151 (53%) |
||||||||||||||
|
STOP‐Bang score ≥ 3 |
206 (72%) |
||||||||||||||
|
OSA‐50 score ≥ 5 |
236 (82%) |
||||||||||||||
|
Epworth sleepiness scale score, mean (SD) |
6.3 (4.2) |
||||||||||||||
|
Epworth sleepiness scale score ≥ 8 |
101 (35%) |
||||||||||||||
|
|
|||||||||||||||
|
SD = standard deviation. ◆ |
|||||||||||||||
Box 3 – Receiver operator characteristic curves for detection of clinically relevant obstructive sleep apnoea* by screening questionnaire scores
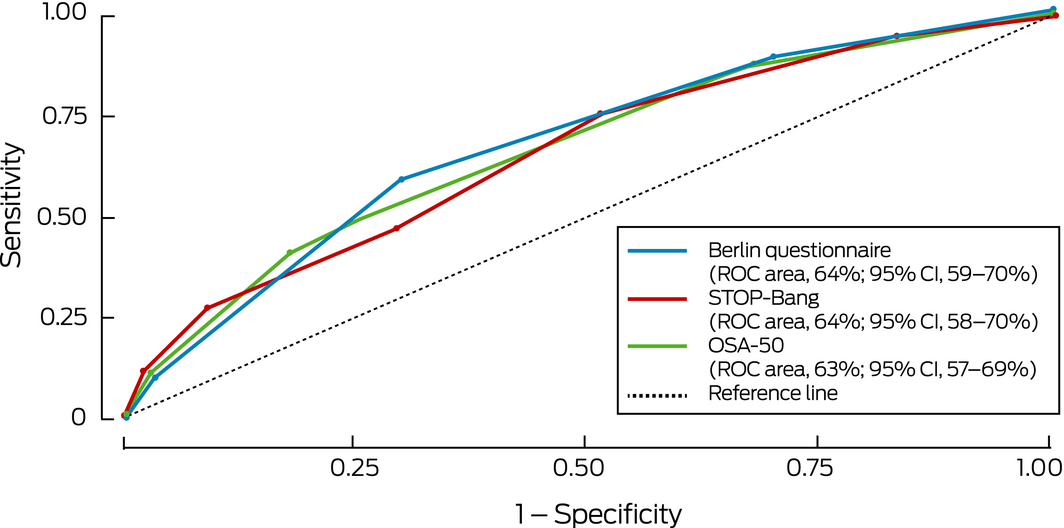
* Defined as moderate to severe obstructive sleep apnoea (oxygen desaturation index ≥ 15) or mild obstructive sleep apnoea (oxygen desaturation index, 5–14) with excessive day time sleepiness (Epworth sleepiness scale score ≥ 8). ◆
Box 4 – Diagnostic utility of obstructive sleep apnoea screening questionnaires, alone and in combination with an Epworth sleepiness scale score of at least 8 for identifying participants with clinically relevant obstructive sleep apnoea* in people with at least one trigger symptom†
|
Screening tests |
Area under ROC curve |
Sensitivity |
Specificity |
Positive predictive value |
Negative predictive value |
Positive likelihood ratio§ |
Negative likelihood ratio§ |
Diagnostic odds ratio§ |
|||||||
|
|
|||||||||||||||
|
Obstructive sleep apnoea screening questionnaires‡ |
|||||||||||||||
|
Berlin |
62% |
65% |
59% |
61% |
63% |
1.6 |
0.6 |
2.7 |
|||||||
|
STOP‐Bang |
58% |
81% |
36% |
55% |
65% |
1.2 |
0.5 |
2.3 |
|||||||
|
OSA‐50 |
54% |
86% |
21% |
52% |
61% |
1.1 |
0.6 |
1.7 |
|||||||
|
Obstructive sleep apnoea screening questionnaires‡ and Epworth sleepiness scale score ≥ 8 |
|||||||||||||||
|
Berlin |
66% |
36% |
95% |
88% |
60% |
7.3 |
0.7 |
10.9 |
|||||||
|
STOP‐Bang |
71% |
50% |
92% |
86% |
65% |
6.4 |
0.5 |
11.7 |
|||||||
|
OSA‐50 |
72% |
51% |
92% |
87% |
66% |
6.6 |
0.5 |
12.4 |
|||||||
|
|
|||||||||||||||
|
CI = confidence interval; ROC = receiver operator characteristic. * Defined as moderate to severe obstructive sleep apnoea (oxygen desaturation index ≥ 15) or mild obstructive sleep apnoea (oxygen desaturation index, 5–14) with excessive day time sleepiness (Epworth sleepiness scale score ≥ 8). † Troublesome snoring, witnessed apnoeas, or sleepiness or fatigue/tiredness (online Supporting Information, table 1). ‡ Standard questionnaire cut‐off scores were applied. § The positive likelihood ratio — sensitivity/(1 – specificity) — compares the probability of a positive test result for someone with the disorder with that for someone without the disorder; the negative likelihood ratio — (1 – sensitivity)/specificity — compares the probabilities of a negative result for people with and without the disorder. The ratio of the positive and negative likelihood ratios is the diagnostic odds ratio, a measure of the overall accuracy of the test. ◆ |
|||||||||||||||
Box 5 – Receiver operator characteristic curves for detection of clinically relevant obstructive sleep apnoea* by screening questionnaire scores combined with Epworth sleepiness scale score ≥ 8
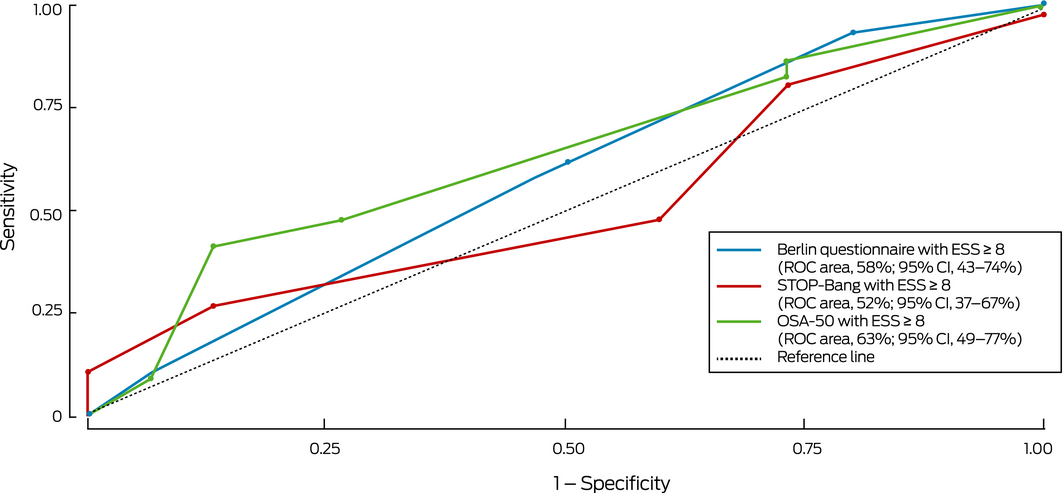
* Defined as moderate to severe obstructive sleep apnoea (oxygen desaturation index ≥ 15) or mild obstructive sleep apnoea (oxygen desaturation index, 5–14) with excessive day time sleepiness (Epworth sleepiness scale score ≥ 8). ◆
Box 6 – A decision support tool for primary care: utility of using different STOP‐Bang scores, alone or in combination with Epworth sleepiness scale (ESS) scores for detecting clinically relevant obstructive sleep apnoea*
|
STOP‐Bang and ESS cut‐off scores |
Patients with clinically relevant OSA excluded if criteria used to rule out clinically relevant disease |
Healthy persons included for further assessment if criteria used to rule in clinically relevant disease |
|||||||||||||
|
|
|||||||||||||||
|
STOP‐Bang score alone |
|||||||||||||||
|
≥ 2 |
2% |
96% |
|||||||||||||
|
≥ 3 |
19% |
64% |
|||||||||||||
|
≥ 4 |
48% |
38% |
|||||||||||||
|
≥ 5 |
69% |
12% |
|||||||||||||
|
≥ 6 |
86% |
3% |
|||||||||||||
|
≥ 7 |
97% |
0 |
|||||||||||||
|
STOP‐Bang score and ESS < 8 |
|||||||||||||||
|
≥ 2 |
2% |
95% |
|||||||||||||
|
≥ 3 |
19% |
63% |
|||||||||||||
|
≥ 4 |
42% |
34% |
|||||||||||||
|
≥ 5 |
62% |
12% |
|||||||||||||
|
≥ 6 |
83% |
3% |
|||||||||||||
|
≥ 7 |
94% |
0 |
|||||||||||||
|
STOP‐Bang score and ESS ≥ 8 |
|||||||||||||||
|
≥ 2 |
39% |
11% |
|||||||||||||
|
≥ 3 |
50% |
8% |
|||||||||||||
|
≥ 4 |
70% |
6% |
|||||||||||||
|
≥ 5 |
86% |
1% |
|||||||||||||
|
≥ 6 |
94% |
0 |
|||||||||||||
|
≥ 7 |
99% |
0 |
|||||||||||||
|
|
|||||||||||||||
|
* Defined as moderate to severe obstructive sleep apnoea (oxygen desaturation index ≥ 15) or mild obstructive sleep apnoea (oxygen desaturation index, 5–14) with excessive day time sleepiness (Epworth sleepiness scale score ≥ 8). ◆ |
|||||||||||||||
Received 25 November 2018, accepted 12 February 2019
- Chamara V Senaratna1,2
- Jennifer L Perret3
- Adrian Lowe1
- Gayan Bowatte1
- Michael J Abramson4
- Bruce Thompson5
- Caroline Lodge1
- Melissa Russell1
- Garun S Hamilton4,6
- Shyamali C Dharmage7
- 1 Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC
- 2 University of Sri Jayewardenepura, Nugegoda, Sri Lanka
- 3 Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC
- 4 Monash University, Melbourne, VIC
- 5 The Alfred Hospital, Melbourne, VIC
- 6 Monash Lung and Sleep Institute, Monash Health, Melbourne, VIC
- 7 Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, University of Melbourne, Melbourne, VIC
We acknowledge the National Health and Medical Research Council (NHMRC) for funding (299901, 10212750) and the Australian Department of Education for graduate training and scholarships (Chamara Senaratna). We also thank ResMed for providing some of the ApneaLink devices. We thank Frances Chung and the University Health Network of Canada for their permission to use the STOP‐Bang questionnaire (www.stopbang.ca).
Adrian Lowe and Jennifer Perret received grants from the NHMRC during the conduct of the study; Michael Abramson received grants from Pfizer and Boehringer–Ingelheim, and conference attendance support and personal consultancy fees from Sanofi for activities not related to this article. Garun Hamilton received equipment for research purposes from ResMed, Philips Respironics, and Air Liquide Healthcare for activities not related to this article; Garun Hamilton was a member of the Thoracic Medicine Clinical Committee of the MBS Review process. Jennifer Perret received fellowship funding from the NHMRC during the period of the study.
- 1. Senaratna CV, Perret JL, Lodge C, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017; 34: 70–81.
- 2. Leger D, Bayon V, Laaban JP, Philip P. Impact of sleep apnea on economics. Sleep Med Rev 2012; 16: 455–462.
- 3. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317: 415–433.
- 4. Kapur V, Strohl KP, Redline S, et al. Underdiagnosis of sleep apnea syndrome in US communities. Sleep Breath 2002; 6: 49–54.
- 5. Stewart SA, Penz E, Fenton M, Skomro R. Investigating cost implications of incorporating level III at‐home testing into a polysomnography based sleep medicine program using administrative data. Can Respir J 2017; 2017: 1–7.
- 6. Kunisaki KM, Khalil W, Koffel E, et al. The comparative effectiveness, harms, and cost of care models for the evaluation and treatment of obstructive sleep apnea (OSA): a systematic review (Evidence‐based Synthesis Program, no. 09‐009). Washington (DC): Department of Veterans Affairs (US), 2016; pp. 1–12. https://www.ncbi.nlm.nih.gov/books/NBK441790 (viewed July 2018).
- 7. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545.
- 8. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131: 485–491.
- 9. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108: 812–821.
- 10. Chai‐Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax 2011; 66: 213–219.
- 11. Sánchez‐Quiroga MÁ, Corral J, Gómez‐de‐Terreros FJ, et al; the Spanish Sleep Network and Primary Care Group. Primary care physicians can comprehensively manage sleep apnea patients: a non‐inferiority randomized controlled trial. Am J Respir Crit Care Med 2018; 198: 648–656.
- 12. Medicare Benefits Schedule Review Taskforce. Final report from the Thoracic Medicine Clinical Committee. Canberra: Department of Health, 2016. http://www.health.gov.au/internet/main/publishing.nsf/content/C195FC32CB2DBAD6CA25801800175428/$File/MBS%20Thoracic%20Report%20FINAL.pdf (viewed July 2018).
- 13. Matheson MC, Abramson MJ, Allen K, et al; TAHS investigator group. Cohort profile: the Tasmanian Longitudinal Health STUDY (TAHS). Int J Epidemiol 2017; 46: 407–408i.
- 14. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373: 82–93.
- 15. Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 2010; 24: 843–851.
- 16. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005; 112: 2660–2667.
- 17. Gantner D, Ge JY, Li LH, et al. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology 2010; 15: 952–960.
- 18. Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse‐led care for symptomatic moderate–severe obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179: 501–508.
- 19. Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med 2012; 23: 586–593.
- 20. Wald N, Bestwick J. Is the area under an ROC curve a valid measure of the performance of a screening or diagnostic test? J Med Screen 2014; 21: 51–56.
- 21. Chung F, Subramanyam R, Liao P, et al. High STOP‐Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012; 108: 768–775.
- 22. Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161: 210–220.
- 23. Ng Y, Joosten SA, Edwards BA, et al. Oxygen desaturation index differs significantly between types of sleep software. J Clin Sleep Med 2017; 13: 599–605.
- 24. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep‐disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001; 163: 608–613.
- 25. Malhotra RK, Kirsch DB, Kristo DA, et al. Polysomnography for obstructive sleep apnea should include arousal‐based scoring: an American Academy of Sleep Medicine position statement. J Clin Sleep Med 2018; 14: 1245–1247.





Abstract
Objective: To examine the utility of apnoea screening questionnaires, alone and in combination with the Epworth sleepiness scale (ESS), for detecting obstructive sleep apnoea (OSA) in primary care.
Design, setting: Prospective validation study in an Australian general population cohort.
Participants: 424 of 772 randomly invited Tasmanian Longitudinal Health Study, 6th decade follow‐up participants with OSA symptoms (mean age, 52.9 years; SD, 0.9 year) who completed OSA screening questionnaires and underwent type 4 sleep studies.
Main outcome measures: Clinically relevant OSA, defined as moderate to severe OSA (15 or more oxygen desaturation events/hour), or mild OSA (5–14 events/hour) and excessive daytime sleepiness (ESS ≥ 8); diagnostic test properties of the Berlin (BQ), STOP‐Bang and OSA‐50 questionnaires, alone or combined with an ESS ≥ 8.
Results: STOP‐Bang and OSA‐50 correctly identified most participants with clinically relevant OSA (sensitivity, 81% and 86% respectively), but with poor specificity (36% and 21% respectively); the specificity (59%) and sensitivity of the BQ (65%) were both low. When combined with the criterion ESS ≥ 8, the specificity of each questionnaire was high (94–96%), but sensitivity was low (36–51%). Sensitivity and specificity could be adjusted according to specific needs by varying the STOP‐Bang cut‐off score when combined with the ESS ≥ 8 criterion.
Conclusions: For people likely to trigger OSA assessment in primary care, the STOP‐Bang, BQ, and OSA‐50 questionnaires, combined with the ESS, can be used to rule in, but not to rule out clinically relevant OSA. Combined use of the STOP‐Bang with different cut‐off scores and the ESS facilitates a flexible balance between sensitivity and specificity.