The known Guidelines for the management of COPD recommend spirometry testing when diagnosing COPD. Smoking cessation reduces the risk of developing COPD and slows lung function decline.
The new More than one-third of participants managed for COPD did not meet the spirometric definition of the disorder, while one in six participants not previously diagnosed with COPD had spirometry test results consistent with COPD.
The implications Case finding and effective use of spirometry could improve the diagnosis of COPD in primary care. Health professionals need to understand the smoking cessation support preferences of smokers, offer evidence-based advice, and closely monitor any difficulties or side effects during quit attempts.
Chronic obstructive pulmonary disease (COPD) is globally a major public health problem that has significant effects on mortality, morbidity, and health resource utilisation; it is the fifth leading cause of death in Australia.1 The prevalence of moderate to severe COPD is 7.5% among Australians aged 40 years or more, and 29.2% among those aged 75 years or more.2
COPD is characterised by airflow limitation. The Global Initiative for Obstructive Lung Disease (GOLD)3 and the national COPD-X guidelines4 each advocate using spirometry for diagnosing COPD, but it is not undertaken in primary care settings as frequently as recommended.5 This may contribute to delaying diagnosis of COPD and the treatment of patients at high risk.
Tobacco smoking is the most preventable risk factor for COPD. Although the overall prevalence of smoking in Australia has declined, 12% of people aged 14 years or more smoke daily.6 As many as 50% of smokers develop clinically significant COPD.7 Smoking cessation is the key to preventing and treating COPD.
About one in five patients attending general practices is a smoker.8 Clinic visits provide good opportunities for general practitioners to review respiratory symptoms and exposure to risk factors, to suggest changes in smoking behaviour, and to provide advice about cessation strategies, potentially reducing the risk of COPD. Knowledge of the smoking experiences and cessation preferences of smokers can inform individualised cessation interventions that can be sustained by those attempting to quit. GPs are also well placed to identify patients at higher risk of COPD.5 The optimal application of case-finding approaches and diagnostic tools, including spirometry, can improve the diagnosis and management of COPD in primary care.
In this study, we reviewed the accuracy of COPD diagnoses in primary care, and explored the quitting experiences and preferences of smokers attending general practice clinics.
Methods
The Review of Airway Dysfunction and Interdisciplinary Community-based care in Adult Long-term Smokers (RADICALS)9 study is an ongoing cluster randomised controlled trial assessing an interdisciplinary model of care for reducing the burden of COPD and smoking in Australian primary care. Baseline data were obtained from participants enrolled between February 2015 and April 2017.
Recruitment of and data collection from clinics and participants
The trial protocol has been reported elsewhere.9 Briefly, 43 general practice clinics in Melbourne were recruited with the assistance of primary health networks and key informants, and by direct recruitment. After obtaining signed agreement, clinics were randomised to the control or intervention arms of the study. For the purposes of this analysis, baseline data from the two groups have been combined. Two clinics withdrew from the study before they commenced recruiting participants.
At each clinic, a research assistant searched the practice database to identify eligible patients, and contacted them by mail or telephone to seek their participation. Patients were invited if they were at least 40 years old and had visited a participating clinic at least twice during the previous 12 months, reported being a current or ex-smoker with a smoking history of at least 10 pack-years, or were being managed for COPD. Patients who had a documented diagnosis of COPD or were currently treated with COPD-specific medications (muscarinic antagonists or long-acting muscarinic antagonist/long-acting β-adrenergic agonist combination therapies), were deemed to have a prior diagnosis of COPD. Those with no history of smoking were also eligible if they had spirometry-confirmed COPD or were treated with COPD-specific medications. After providing written informed consent, eligible participants were interviewed at the practice (one hour). Baseline demographic and clinical data were collected, followed by case-finding procedures and referral for spirometry, if indicated.10
Case finding, spirometry and COPD questionnaires
During the baseline interview, forced expiratory volume in 1 second/forced expiratory volume in 6 seconds (FEV1/FEV6) was measured with the COPD-6 device (Vitalograph) according to Lung Foundation Australia recommendations.10 Patients with an FEV1/FEV6 value below 0.75, or who found COPD-6 testing difficult, were referred for spirometry testing and assessment of health-related quality of life, dyspnoea, and symptoms with the St George’s Respiratory Questionnaire (SGRQ),11 the modified Medical Research Council (mMRC) dyspnoea scale,12 and the COPD Assessment Test (CAT).13 If results from recent spirometry testing undertaken outside the trial were available, they were also assessed.
Pre- and post-bronchodilator spirometry testing was performed with Easy on-PC spirometers (ndd Medizintechnik) by trained research assistants in accordance with the American Thoracic Society/European Respiratory Society guidelines.14 Post-bronchodilator spirometry testing was performed 10–15 minutes after 400 μg salbutamol was delivered by a metered dose inhaler and spacer. Best efforts at forced expiration were selected according to the spirometer algorithm. The COPD diagnosis was deemed to be confirmed if the post-bronchodilator FEV1/forced vital capacity (FVC) ratio was less than 0.7. We devised an algorithm with the assistance of respiratory scientists to guide research assistants in determining whether spirometry test results were consistent with COPD (online Appendix 1). If results were ambiguous and required further interpretation, the report was sent to a respiratory scientist or respiratory physician. Patients were defined as having a misdiagnosis if they had been treated for COPD or had a documented diagnosis of COPD, but did not meet the spirometric definition of COPD.
Smoking
Data collected from smokers (regardless of whether they had COPD) included smoking status (according to both self-report and exhaled carbon monoxide testing), age at which they started smoking, years of smoking, smoking-related behaviour, pharmacological and non-pharmacological strategies tried during attempts to quit in the preceding 12 months, side effects of previous pharmacotherapy, and difficulties associated with previous attempts to quit. The participants’ preferred methods of smoking cessation for future attempts and their motivation for and confidence in giving up smoking were also assessed.
Statistical analysis
Data were entered into an Access (Microsoft) database, and analysed in Excel (Microsoft) and SPSS 24.0 (IBM). The characteristics of the recruited clinics and the baseline characteristics of participants were summarised as means (with standard deviations [SDs]), medians (with interquartile ranges [IQRs]), or numbers (and percentages), depending on the type and distribution of data. The characteristics (age, sex, country of birth, language spoken at home, education, employment status, marital status, current living arrangements, smoking status, spirometric confirmation of COPD) of those previously diagnosed with or treated for COPD were compared with those of participants without a prior COPD diagnosis. Age, sex, current smoking status, SGRQ score, mMRC grade, CAT score, FEV1/FVC ratio, and the severity of disease in incident cases of COPD were also compared with those of participants with a prior diagnosis of COPD. The statistical significance of differences between groups was assessed in χ2 or Fisher exact tests (categorical variables), Student t tests (continuous variables) or Mann–Whitney U tests (ordinal variables). Confidence intervals (CIs) for proportions were estimated with the exact binomial distribution. P < 0.05 (two-sided) was deemed statistically significant.
Ethics approval
This project was approved by the Monash University Human Research Ethics Committee and the La Trobe University Human Ethics Committee (reference, CF14/1018–2014000433).
Results
The characteristics of the recruited clinics are summarised in Box 1. Spirometers were available in 16 of 43 practices. Many clinics had had no staff trained in spirometry testing, smoking cessation, or COPD management in the previous 2 years.
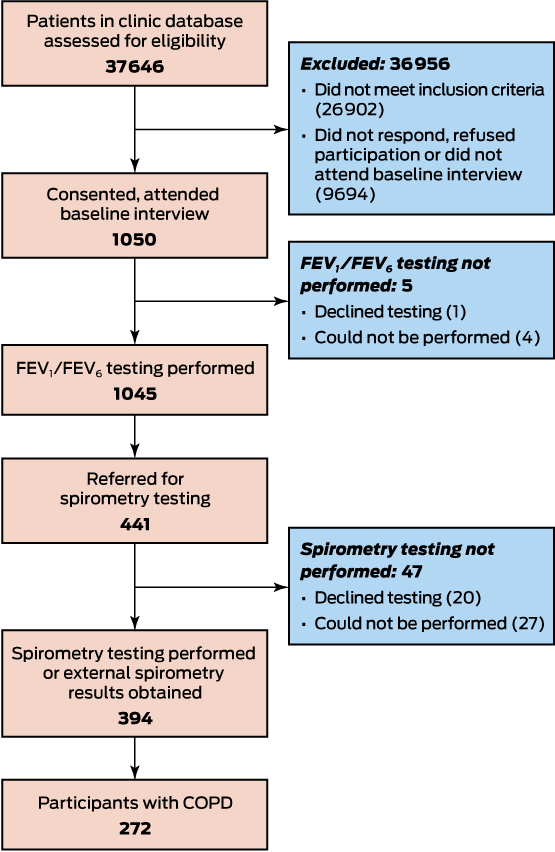
Of the 37 646 patients screened, 10 744 satisfied the inclusion criteria; of these, 1050 (9.8%) attended baseline interviews at 41 clinics (Box 2; demographic and clinical characteristics: online Appendix 2, table 1). Almost all participants underwent FEV1/FEV6 testing during their baseline interview (1045, 99.5%); 272 (25.9% of all participants [95% CI, 23.3–28.7%] and 69.0% [95% CI, 64.2–73.6%] of those who underwent spirometry) had COPD according to their spirometry test results.
Of the 245 participants with a prior COPD diagnosis, 91 (37%) did not meet the spirometric definition of COPD or have a clinical correlation (Box 3): 68 did not meet the criteria for spirometry referral after COPD-6 testing, the spirometry results for 14 were inconsistent with COPD after further interpretation or clinical correlation, those for eight were within normal limits, and the result for one participant was inconsistent with the spirometry-based definition of COPD, but possibly reflected a reversible obstructive disorder, such as asthma. Further, 142 of 805 participants without a prior COPD diagnosis (17.6%; 95% CI, 15.1–20.5%) had spirometry results consistent with COPD (Box 3). The characteristics of 142 participants with prior COPD diagnoses (prevalent cases) and 130 with new diagnoses confirmed by spirometry (incident cases) are compared in Box 4.
Of the 1050 participants who attended a baseline interview, 690 (65.7%) were current smokers, most of whom smoked daily (646, 93.6% of smokers) (Box 5, Box 6). Of the current smokers, 360 (52.2%) had attempted to quit at least once during the previous 12 months. The pharmacological treatments most frequently tried were nicotine replacement therapy (205, 57.4%) and varenicline (110, 30.8%) (Box 7). Non-evidence-based treatments, including hypnotherapy (62, 17%) and electronic cigarettes (38, 11%), were also frequently tried. Most smokers (286, 81.0%) reported difficulties during past attempts to quit, including urges to smoke (195, 55.2%) and irritability or aggression (152, 43.1%) (online Appendix 2, table 2). Of the 242 smokers who reported using cessation medications, more than half (157, 68.9%) reported side effects (data not shown).
Current smokers would consider a variety of strategies in future attempts to quit. Pharmacotherapy was the most popular (272, 39.9%); e-cigarettes would be considered by more than one-quarter of smokers (187, 27.6%) (online Appendix 2, table 3).
Discussion
More than one-third of participants with a prior diagnosis of COPD did not meet the spirometric definition of the disorder, while one in six participants not previously diagnosed with COPD had spirometry test results consistent with COPD. The mean age of patients with a prior diagnosis of COPD was higher than for those without an existing COPD diagnosis; the proportions of patients in this group with lower education levels, without fulltime employment, without a partner, or living alone were also larger than among participants without an existing diagnosis (online Appendix 2, table 1). The mean age for incident cases of COPD was lower and the disease severity milder than for prevalent cases; lung function was better in incident cases, and SGRQ and CAT scores were lower. The proportion of patients with a prior diagnosis of COPD who were current smokers (44%) was significantly smaller than that of those newly diagnosed on the basis of spirometry test results (77%), and was also smaller than the proportion of all participants without a prior COPD diagnosis (72%), but still high. A slight majority of current smokers had unsuccessfully attempted to quit during the previous year; difficulties during these attempts were common. More than half of those attempting to quit reported using nicotine replacements or varenicline; side effects were frequent. Interest in non-evidence-based smoking cessation strategies was high.
Our study highlights the importance of applying evidence-based guidelines to COPD management and smoking cessation support in Australian primary care. COPD diagnosis and management guidelines are widely disseminated in Australia,15 but diagnostic spirometry is not employed appropriately. In an earlier study in Australian primary care, only 58% of patients receiving treatment for COPD met the spirometric criteria for the disorder.16 Lack of access to a spirometer, inadequate training in performing spirometry and interpreting the results, and poor remuneration are among the many challenges for lung function testing in primary care.16,17 Lack of awareness of the need for spirometry testing for diagnosing COPD may lead to diagnoses based on social history, symptoms, or chest x-rays alone. Misdiagnosis can lead to unnecessary or inappropriate treatment, potentially with adverse consequences. Underdiagnosis delays initiation of lifestyle changes and targeted therapy, leading to an increased risk of exacerbations and pneumonia.18
The approach to diagnosing COPD in Australia is reactive; formal diagnosis follows the initial presentation of symptoms by patients, and there is inadequate emphasis on case finding in smokers.19 Our case-finding approach — FEV1/FEV6 testing of high risk patients followed by spirometry testing and symptom questionnaires — identified 142 new cases of COPD that may not have been recognised until symptoms developed as the disease progressed.
Recommendations about screening for early identification of COPD are inconsistent. The United States Preventive Service Task Force (USPSTF) found no overall benefit in spirometry-based screening of asymptomatic individuals.20 However, the National Institute for Health and Care Excellence (NICE) guidelines recommend opportunistic case finding based on risk factors such as age, smoking, and symptoms.21 Lung Foundation Australia recommends using symptom questionnaires to assist with COPD case finding and diagnosis, especially in conjunction with FEV1/FEV6 assessment, for which an upper threshold of 0.75 is highly sensitive and specific.22 All case-finding approaches increase the detection of COPD in primary care, although the effects on clinical care and patient outcomes need to be further investigated.23
The reported rates of pharmacological and non-pharmacological agent use in our study were similar to those found by an earlier study of smoking cessation aids used by smokers admitted to three Victorian hospitals,24 in which nicotine replacement therapy was also the most frequently used pharmacotherapy and hypnotherapy the most common non-pharmacological therapy. Combination nicotine replacement therapy and varenicline are the most effective quitting aids, and are of similar efficacy.25 In our study, only one-third of current smokers reported trying varenicline, despite strong evidence for its effectiveness.25 Further, it is reported that few smokers continue pharmacotherapy for the recommended duration of treatment.24
Nicotine replacement therapy is widely available from pharmacies and supermarkets, while varenicline is a prescription-only drug, requiring a visit to a GP and Pharmaceutical Benefits Scheme authority approval. Many of our participants reported interest in using medications in future attempts to quit, but more than one-quarter would also consider going “cold turkey”. As the success rate of unassisted cessation is low, it should be recommended to all smokers interested in quitting that they seek assistive pharmacotherapy, together with counselling and support, unless there are contraindications.26 Consultations with smokers in general practice provide opportunities for counselling and motivational support, crucial to successful quitting. The Royal Australian College of General Practitioners (RACGP) guidelines (specifically, the 5As structure: ask, assess, advise, assist, and arrange follow-up) should guide smoking cessation support.26
Smokers frequently try non-evidence-based methods for quitting. Although hypnotherapy was popular with our participants, evidence for its efficacy is scant.27 The degree to which participants have used e-cigarettes as cessation aids and the relatively high interest in trialling them in future attempts is worrying. The efficacy of e-cigarettes as a smoking cessation aid and their long term safety are unknown.28 Despite widespread popularity in some countries, e-cigarettes are not approved by the Therapeutic Goods Administration, nor are they recommended by the National Health and Medical Research Council as a cessation aid.29
We recruited a large sample of participants from a diverse range of practices across Melbourne (including specialised clinics serving people from lower socio-demographic status areas, and drug and alcohol addiction clinics). The smoking status of participants was objectively confirmed by exhaled carbon monoxide testing when possible. However, we also relied upon participant reports, which are subject to recall bias. Response and social desirability bias may have also influenced survey responses.
As with other general practice studies of smokers and COPD populations, the participation rate was modest (10%), possibly affecting our estimates of prevalence and incidence rates and the generalisability of our results. However, the characteristics of our participants were similar to those of a group recruited for a comparable study across 44 general practices in Sydney that found similar rates of COPD misdiagnosis.16 Patients who volunteered for the trial may have been more motivated than those who did not, and may therefore not be representative of all eligible patients. We could not verify the self-reported use of smoking cessation aids in medical records or dispensing and prescribing data, but the research assistants responsible for collecting information were practising pharmacists familiar with smoking cessation medications and in taking medication histories. Some patients used their COPD medications on the day of spirometry testing, possibly causing detection errors.
Conclusions and implications for practice
Evidence-based guidelines for the optimal diagnosis and management of COPD must be actively promoted and implemented in Australian primary care. GPs and other primary care practitioners should be educated about the role of spirometry testing in COPD diagnosis, and provided with resources and incentives for adopting it in practice. Early identification of potential COPD in individuals at high risk by case finding may facilitate earlier diagnosis and initiation of treatment.
In accordance with RACGP guidelines, smoking cessation strategies of proven effectiveness should be recommended to all smokers. Patients may ask health professionals about the efficacy and safety of non-evidence-based methods, including e-cigarettes, hypnotherapy and acupuncture; e-cigarettes, in particular, should not be recommended. Past experience with attempts to quit, reasons for relapse, and preferences for future smoking cessation attempts should inform recommendations about cessation and assessment of the need for monitoring.
Box 1 – Characteristics of the 43 recruited clinics*
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Type of practice |
|
||||||||||||||
|
Single GP practice |
6 (14%) |
||||||||||||||
|
Group GP practice/community health centre/interdisciplinary practice |
37 (86%) |
||||||||||||||
|
Number of GPs, median (IQR) |
5 (4–10) |
||||||||||||||
|
Number of patients on database, median (IQR) |
8214 (2000–18 309) |
||||||||||||||
|
Spirometer available |
16 (43%) |
||||||||||||||
|
Staff training in the previous 2 years |
|
||||||||||||||
|
Spirometry |
13 (35%) |
||||||||||||||
|
Smoking cessation |
6 (17%) |
||||||||||||||
|
COPD management |
7 (20%) |
||||||||||||||
|
|
|||||||||||||||
|
COPD = chronic obstructive pulmonary disease; IQR = interquartile range. * Two practices withdrew from the study after enrolment and before participant recruitment. Some data are missing for all variables, except for practice type and number of GPs. |
|||||||||||||||
Box 3 – Spirometric confirmation of chronic obstructive pulmonary disease (COPD) by spirometry test results or clinical correlation
|
|
All participants |
No prior diagnosis of COPD |
Prior diagnosis of COPD |
P |
|||||||||||
|
|
|||||||||||||||
|
Number of participants |
1050 |
805 |
245 |
|
|||||||||||
|
Number referred for spirometry |
441 (42.0%) |
264 (32.8%) |
177 (72.2%) |
|
|||||||||||
|
Number who underwent spirometry |
394 (37.5%) |
234 (29.1%) |
160 (65.3%) |
|
|||||||||||
|
COPD confirmed |
272 (25.9%) |
142 (17.6%) |
130 (53.1%) |
< 0.001 |
|||||||||||
|
COPD not confirmed |
716 (68.2%) |
625 (77.6%) |
91 (37%) |
||||||||||||
|
No result* |
62 (5.9%) |
38 (4.7%) |
24 (9.8%) |
||||||||||||
|
|
|||||||||||||||
|
* It was not possible to obtain interpretable results from 62 participants referred for spirometry testing (uncontactable or declined spirometry, 47; inconclusive results, 15). |
|||||||||||||||
Box 4 – Characteristics of participants with prior and new diagnoses of chronic obstructive pulmonary disease (COPD) confirmed by spirometry testing
|
Characteristic |
Incident cases (no prior COPD diagnosis) |
Prevalent cases (prior COPD diagnosis) |
P |
||||||||||||
|
|
|||||||||||||||
|
Number of participants |
142 |
130 |
|
||||||||||||
|
Age (years), mean (SD) |
62.0 (10.7) |
67.4 (10.2) |
< 0.001 |
||||||||||||
|
Sex (men) |
89 (63%) |
78 (60%) |
0.65 |
||||||||||||
|
Currently smoking |
109 (77%) |
57 (44%) |
< 0.001 |
||||||||||||
|
SGRQ score,* mean (SD) |
26.3 (16.5) |
38.6 (17.7) |
< 0.001 |
||||||||||||
|
mMRC grade,† median (IQR) |
1 (0–1) |
1 (1–2) |
< 0.001 |
||||||||||||
|
CAT score,‡ mean (SD) |
11.3 (7.0) |
15.3 (7.9) |
< 0.001 |
||||||||||||
|
Post-bronchodilator FEV1/FVC ratio, median (IQR)§ |
0.62 (0.56–0.67) |
0.56 (0.46–0.62) |
< 0.001 |
||||||||||||
|
Severity of disease§,¶ |
|
|
< 0.001 |
||||||||||||
|
Mild |
117 (83.6%) |
73 (57%) |
|
||||||||||||
|
Moderate |
16 (11%) |
40 (31%) |
|
||||||||||||
|
Severe |
7 (5%) |
16 (12%) |
|
||||||||||||
|
|
|||||||||||||||
|
FEV1/FVC = forced expiratory volume in 1 second/forced vital capacity ratio; IQR = interquartile range; SD = standard deviation. * St George’s Respiratory Questionnaire: 50-item questionnaire for assessing health status in patients with diseases of airway obstruction; range, 0–100; higher scores indicate lower health-related quality of life. Data missing for 14 participants (six incident, eight prevalent cases). † Modified Medical Research Council dyspnoea scale: scale consisting of five statements; range, 0 (no dyspnoea) to 4 (very severe dyspnoea). Data missing for one incident case. ‡ COPD Assessment Test: eight-item questionnaire for assessing health status of patients with COPD; range, 0–40; higher scores indicate poorer health. Data missing for one incident case. § Data missing for three participants (one prevalent and two incident cases) because only pre-bronchodilator spirometry was performed. ¶ Severity of COPD based on FEV1% predicted values (mild, 60–80% predicted; moderate, 40–59% predicted; severe, < 40% predicted).4 Eighty participants had FEV1% predicted values of more than 80, but had characteristic symptoms of mild COPD. |
|||||||||||||||
Box 5 – Characteristics of the 1050 participants who completed the baseline interview
|
Characteristic |
Total participants |
No prior diagnosis of COPD |
Prior diagnosis of COPD |
P |
|||||||||||
|
|
|||||||||||||||
|
Number of participants |
1050 |
805 |
245 |
|
|||||||||||
|
Age (years), mean (SD) |
60.5 (11.1) |
58.5 (10.4) |
67.1 (10.6) |
< 0.001 |
|||||||||||
|
Sex (men) |
564 (53.7%) |
433 (53.8%) |
131 (53.5%) |
0.93 |
|||||||||||
|
Born in Australia* |
735 (70.3%) |
571 (71.2%) |
164 (67.5%) |
0.27 |
|||||||||||
|
Current smokers |
690 (65.7%) |
582 (72.3%) |
108 (44.0%) |
< 0.001 |
|||||||||||
|
Daily smokers |
646 (61.5%) |
542 (67.3%) |
104 (42.4%) |
< 0.001 |
|||||||||||
|
Occasional smokers |
44 (4.2%) |
40 (5.0%) |
4 (1.6%) |
0.022 |
|||||||||||
|
Ex-smokers |
350 (33.3%) |
222 (27.6%) |
128 (52.2%) |
< 0.001 |
|||||||||||
|
Never smokers |
10 (1.0%) |
1 (0.1%) |
9 (3.7%) |
< 0.001 |
|||||||||||
|
|
|||||||||||||||
|
COPD = chronic obstructive pulmonary disease; SD = standard deviation. * Data missing for five participants. Additional demographic data is included in online Appendix 2, table 1. |
|||||||||||||||
Box 6 – Characteristics of the 690 participants who were current smokers
|
Characteristic |
|
||||||||||||||
|
|
|||||||||||||||
|
Number of years of smoking, mean (SD) |
37.2 (11.0) |
||||||||||||||
|
Exhaled carbon monoxide level* (ppm), median (IQR) |
21 (13–28) |
||||||||||||||
|
Motivation to give up smoking, median (IQR)† |
6 (4–8) |
||||||||||||||
|
Confidence in giving up smoking, median (IQR)‡ |
5 (3–7) |
||||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. * Exhaled carbon monoxide level below 7 ppm confirms participant is a non-smoker (manufacturer’s recommended cut-off). Carbon monoxide breath tests could not be obtained from six participants; 46 current smokers (self-report) had levels below threshold, 21 non-smokers (self-report) had levels at or above threshold. † Scale, 1 (low) to 10 (high); data missing for six participants. ‡ Scale, 1 (low) to 10 (high); data missing for seven participants. |
|||||||||||||||
Box 7 – Self-reported pharmacological and non-pharmacological treatments by 360 current smokers who had attempted to quit at least once in the previous year
|
Treatment* |
|
||||||||||||||
|
|
|||||||||||||||
|
Evidence-based treatments |
|
||||||||||||||
|
Nicotine replacement therapy |
205 (57.4%) |
||||||||||||||
|
Varenicline |
110 (30.8%) |
||||||||||||||
|
Quitline |
29 (8%) |
||||||||||||||
|
Bupropion |
25 (7%) |
||||||||||||||
|
Non-evidence-based treatments |
|
||||||||||||||
|
Hypnotherapy |
62 (17%) |
||||||||||||||
|
E-cigarettes |
38 (11%) |
||||||||||||||
|
Acupuncture |
21 (6%) |
||||||||||||||
|
Other† |
41 (12%) |
||||||||||||||
|
|
|||||||||||||||
|
* Multiple selections possible; missing data for three participants. † For example, cold turkey, herbal cigarettes, homeopathic remedies, DVDs or books, counselling, Quit smoking group, online program. |
|||||||||||||||
Received 9 July 2017, accepted 15 November 2017
- Jenifer Liang1
- Michael J Abramson2
- Nicholas A Zwar3,4
- Grant M Russell5
- Anne E Holland6,7,8
- Billie Bonevski9
- Ajay Mahal2,10
- Kirsten Phillips11
- Paula Eustace12
- Eldho Paul2,13
- Sally Wilson1
- Johnson George1
- 1 Centre for Medicine Use and Safety, Monash University, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 University of New South Wales, Sydney, NSW
- 4 University of Wollongong, Wollongong, NSW
- 5 Southern Academic Primary Care Research Unit, Monash University, Melbourne, VIC
- 6 La Trobe University, Melbourne, VIC
- 7 Alfred Health, Melbourne, VIC
- 8 Institute for Breathing and Sleep, Austin Hospital, Melbourne, VIC
- 9 University of Newcastle, Newcastle, VIC
- 10 The Nossal Institute for Global Health, University of Melbourne, Melbourne, VIC
- 11 Lung Foundation Australia, Brisbane, QLD
- 12 Eastern Melbourne PHN, Melbourne, VIC
- 13 Alfred Hospital, Melbourne, VIC
This trial is funded by the National Health and Medical Research Council (NHMRC) through the NHMRC Partnerships for Better Health – Partnership Projects initiative (APP1076255). Cash and in-kind contributions were received from our partner organisations, Lung Foundation Australia (LFA), Boehringer Ingelheim, and Eastern Melbourne PHN (EMPHN). The LFA and EMPHN were involved in project design and conduct, and contributed to data analysis and writing of manuscripts. Boehringer Ingelheim was involved in project discussions, planning and progress review, but had no involvement in the design of the intervention program, and did not contribute to decisions about data analysis and the dissemination of findings. Billie Bonevski is supported by an NHMRC Career Development Fellowship (GNT1063206) and a Faculty of Health and Medicine, University of Newcastle, Gladys M. Brawn Career Development Fellowship. Jenifer Liang receives the Cyril Tonkin Scholarship 2014, administered by the Victorian College of Pharmacy Foundation Board, Monash University. We thank Denise van den Bosch (project manager), and all research assistants, clinics and participants.
Johnson George, Billie Bonevski and Michael J Abramson have held an investigator-initiated grant from Pfizer for unrelated research. Michael J Abramson has received assistance for conference attendance from Sanofi. Johnson George and Nicholas A Zwar are members of the Lung Foundation Australia COPD Guidelines Committee; Michael J Abramson was Chair of the committee (2004–14). Anne E Holland is a member of the Lung Foundation Australia COPD-XConcise Guide for Primary Care Advisory Committee. Kirsten Phillips is general manager of the COPD National Program, Lung Foundation Australia.
- 1. Australian Centre for Asthma Monitoring 2011. Asthma in Australia 2011: with a focus chapter on chronic obstructive pulmonary disease (AIHW Cat. No. ACM 22; Asthma Series No. 4). Canberra: AIHW, 2011. http://www.aihw.gov.au/publication-detail/?id=10737420159 (viewed Apr 2017).
- 2. Toelle BG, Xuan W, Bird TE, et al. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust 2013; 198: 144-148. <MJA full text>
- 3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2017 report. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (viewed Oct 2017).
- 4. Yang IA, Dabscheck E, George J, et al. The COPD-X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2017, version 2.50. June 2017. http://copdx.org.au/copd-x-plan/ (viewed Oct 2017).
- 5. Johns DP, Walters JA, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis 2014; 6: 1557-1569.
- 6. Australian Institute of Health and Welfare. Smoking. Updated July 2017. https://www.aihw.gov.au/reports-statistics/behaviours-risk-factors/smoking/overview (viewed Oct 2017).
- 7. Lundback B, Lindberg A, Lindstrom M, et al. Not 15 but 50% of smokers develop COPD? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003; 97: 115-122.
- 8. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2009–10. BEACH: Bettering the Evaluation And Care of Health (AIHW Cat. No. GEP 27; General Practice Series No. 27). Canberra: AIHW, 2010. https://www.aihw.gov.au/reports/primary-health-care/general-practice-activity-in-australia-2009-10/contents/table-of-contents (viewed Nov 2017).
- 9. Liang J, Abramson MJ, Zwar N, et al. Interdisciplinary model of care (RADICALS) for early detection and management of chronic obstructive pulmonary disease (COPD) in Australian primary care: study protocol for a cluster randomised controlled trial. BMJ Open 2017; 7: e016985.
- 10. Lung Foundation Australia. COPD screening using the COPD-6. https://lungfoundation.com.au/wp-content/uploads/2014/02/Instruction-sheet-Piko-6-and-COPD-6.pdf (viewed Oct 2017).
- 11. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321-1327.
- 12. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005-1012.
- 13. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34: 648-654.
- 14. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319-338.
- 15. Yang IA, Brown JL, George J, et al. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust 2017; 207: 436-442. <MJA full text>
- 16. Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust 2011; 195: 168-171. <MJA full text>
- 17. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med 2005; 99: 493-500.
- 18. Colak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med 2017; 5: 426-434.
- 19. Bereznicki B, Walters H, Walters J, et al. Initial diagnosis and management of chronic obstructive pulmonary disease in Australia: views from the coal face. Intern Med 2017; 47: 807-813.
- 20. Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force Recommendation statement. JAMA 2016; 315: 1372-1377.
- 21. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (Clinical guideline CG101). June 2010. https://www.nice.org.uk/guidance/cg101 (viewed July 2017).
- 22. Lung Foundation Australia. The Australian Lung Foundation position paper on the use of COPD screening devices for targeted COPD case finding in community settings, 2011. Feb 2016. http://lungfoundation.com.au/wp-content/uploads/2014/02/Position-Paper.pdf (viewed Aug 2015).
- 23. Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med 2015; 25: 15056.
- 24. Thomas D, Abramson MJ, Bonevski B, et al. Quitting experiences and preferences for a future quit attempt: a study among inpatient smokers. BMJ Open 2015; 5: e006959.
- 25. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; (5): CD009329.
- 26. Supporting smoking cessation: a guide for health professionals. Melbourne: The Royal Australian College of General Practitioners, 2011; updated July 2014. https://www.racgp.org.au/your-practice/guidelines/smoking-cessation/ (viewed Oct 2017).
- 27. Barnes J, Dong CY, McRobbie H, et al. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2010; (10): CD001008.
- 28. Kaisar MA, Prasad S, Liles T, Cucullo L. A decade of e-cigarettes: limited research and unresolved safety concerns. Toxicology 2016; 365: 67-75.
- 29. National Health and Medical Research Council. NHMRC CEO Statement: electronic cigarettes (e-cigarettes) 2017; updated Apr 2017. https://www.nhmrc.gov.au/_files_nhmrc/file/publications/17072_nhmrc_-_electronic_cigarettes-web_final.pdf (viewed Oct 2017).





Abstract
Objectives: To review the accuracy of diagnoses of chronic obstructive pulmonary disease (COPD) in primary care in Australia, and to describe smokers’ experiences with and preferences for smoking cessation.
Design, setting and participants: Patients were invited to participate if they were at least 40 years old and had visited participating general practice clinics in Melbourne at least twice during the previous 12 months, reported being current or ex-smokers with a smoking history of at least 10 pack-years, or were being managed for COPD. Interviews based on a structured questionnaire and case finding (FEV1/FEV6 measurement) were followed, when appropriate, by spirometry testing and assessment of health-related quality of life, dyspnoea and symptoms.
Results: 1050 patients attended baseline interviews (February 2015 – April 2017) at 41 practices. Of 245 participants managed for COPD, 130 (53.1%) met the spirometry-based definition (post-bronchodilator FEV1/FVC < 0.7) or had a clinical correlation; in 37% of cases COPD was not confirmed, and no definitive result was obtained for 9.8% of patients. Case finding and subsequent spirometry testing identified 142 new COPD cases (17.6% of participants without prior diagnosis; 95% CI, 15.1–20.5%). 690 participants (65.7%) were current smokers, of whom 360 had attempted quitting during the previous 12 months; 286 (81.0% of those attempting to quit) reported difficulties during previous quit attempts. Nicotine replacement therapy (205, 57.4%) and varenicline (110, 30.8%) were the most frequently employed pharmacological treatments; side effects were common. Hypnotherapy was the most popular non-pharmacological option (62 smokers, 17%); e-cigarettes were tried by 38 (11%). 187 current smokers (27.6%) would consider using e-cigarettes in future attempts to quit.
Conclusions: COPD was both misdiagnosed and missed. Case finding and effective use of spirometry testing could improve diagnosis. Side effects of smoking cessation medications and difficulties during attempts to quit smoking are common. Health professionals should emphasise evidence-based treatments, and closely monitor quitting difficulties and side effects of cessation aids.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12614001155684.