The known Despite growing recognition of the impact of comorbidities on the care and health outcomes of cancer survivors, data on comorbidities in Australian patients that could inform better policy and practice are limited.
The new The incidence rates of depression, pain/pain–inflammation, osteoporosis, diabetes, cardiovascular disorders, and gastric acid disorders were mildly higher among hormone-dependent breast cancer survivors receiving endocrine therapy than in control groups of age-matched women. The increased risk for cardiovascular conditions, depression and osteoporosis declined after the first year of treatment.
The implications Appropriate models of care are needed to manage the multiple comorbidities of breast cancer survivors.
Breast cancer is the most frequently diagnosed cancer in Australian women. The declining mortality among patients with breast cancer is attributed to screening programs and improved therapies.1 Most breast cancers require additional treatment after surgery, including chemotherapy, radiation therapy, or endocrine therapy.2 About 70% of breast cancers are hormone receptor-positive and benefit from endocrine therapy, and the recommended duration of treatment was recently increased from 5 to 10 years.3 Breast cancer treatments may, however, predispose women to developing chronic illnesses.4-6 As the duration of hormonal cancer treatment increases, managing coexisting chronic conditions will be increasingly important: a greater burden of comorbidities is associated with an increased risk of adverse events and of non-adherence to endocrine therapy, which may reduce survival time.7,8
Improving our knowledge of the pattern of chronic conditions in patients with breast cancer and survivors is therefore important for ensuring effective care. Information about the burden of comorbidities among Australians living with cancer, however, is limited; data on chronic conditions over time is not systematically collected in Australia.
Australia has a universal health care funding system administered by the Department of Human Services, and analysis of routinely collected health claims data, particularly from the Pharmaceutical Benefits Scheme (PBS), is being undertaken with increasing frequency by health care investigators.9 With medication dispensing records serving as surrogates for the presence of comorbidities, analysis of PBS data may offer insights into the epidemiology of chronic conditions.10
The objective of our study was to assess the patterns of comorbidities among Australian women with hormone-dependent breast cancer. Specifically, we compared how frequently selected chronic diseases developed in women with breast cancer receiving endocrine therapy and in women without cancer.
Methods
Data source and study design
We undertook a retrospective, rolling cohort study that allowed secondary analysis of de-identified PBS dispensing claims data for a random 10% sample of Australians across all age groups for the period 1 January 2003 – 31 December 2014. The data covered all medicines subsidised by the PBS for community use, inpatient and outpatient use in private hospitals, and outpatient use in public hospitals, as well as Section 100 medicines (the Highly Specialised Drugs Program). Medicines are coded according to the World Health Organization Anatomical Therapeutic Chemical code and PBS schedule item codes.
Dispensing of endocrine therapy was employed as the proxy measure for identifying individuals with hormone-dependent subtypes of breast cancer. This approach has been validated in Australia.11 Endocrine medications subsidised by the PBS include tamoxifen, anastrozole, letrozole and exemestane. Women for whom endocrine therapy medication was dispensed at least once between January 2004 and December 2011 were identified. We selected incident users to determine when therapy started (the index date). Incident users were defined as women for whom endocrine therapy was first dispensed on or after 1 January 2004, for whom endocrine therapy had not been dispensed during the previous year (1 January – 31 December 2003). We excluded prevalent users (women who had received endocrine therapy during 2003) because we were not able to ascertain when they commenced endocrine therapy. We also excluded women treated with exemestane as the initial endocrine therapy, as this agent is only subsidised for the treatment of metastatic breast cancer, while our study focused on women treated with curative intent. The study population for cases and controls was restricted to concessional beneficiaries, as complete ascertainment of medicine use in this group could be assumed; dispensing of medicines with prices below the general co-payment level was not captured in the PBS dataset before April 2012.
Patients were followed up until a comorbidity of interest developed or until censored at the date of the last available dispensing record (end of study period: 31 December 2014). The date of the last dispensing record served as the end date of follow-up, as dates of death were not available.
Outcome measures
The RxRisk-V model12 was employed as the comorbidity index. The RxRisk-V model is generated from drug dispensing records and includes up to 40 general drug categories (online Appendix, table 1). The predictive validity of the RxRisk-V model for one-year mortality is similar in the Australian setting to that of the Charlson Comorbidity Index.10,13
Comorbidities were identified by the medication groupings in the RxRisk-V model. We assessed the development of eight individual comorbidities that contribute to a high burden of disease in Australia:14 cardiovascular disorders (identified by medications indicated for arrhythmias, heart failure, hypertension, ischaemic heart disease, and angina, and anticoagulant and antiplatelet medicines), depression, diabetes, hyperlipidaemia, gastric acid disorders, osteoporosis, reactive airway diseases, and pain or pain–inflammation. The event was defined as the development of the comorbidity of interest in the time-to-event analysis. Women who already had a comorbidity of interest at or prior to the initiation of endocrine therapy were excluded from the relevant time-to-event analysis.
For each of the eight comorbidities, the women with breast cancer were matched 1:10 by age and sex with a control group of women without cancer (that is, women for whom dispensing of targeted cancer therapy or chemotherapy was not recorded, or women who did not receive endocrine therapy during the study period) and without the comorbidity of interest at baseline (Box 1). An index date (last dispensing record for the year) was assigned to the control group of women that matched the year of endocrine therapy initiation for the corresponding case. To be included in the control group, a woman must have had at least one medication dispensing record during the year in which the index date was assigned (for any PBS-subsidised medicine, including medicines indicated for one of the comorbidities in the RxRisk-V model). This ensured that the women in the control group resided in Australia and had had at least one active health service during the index year.
Baseline comorbidities up to one year prior to the assigned index date were determined and scored with the RxRisk-V model.
Statistical analysis
The development of individual comorbidities over time in the cancer and control groups was compared in a Cox regression analysis, adjusted for the number of baseline comorbidities (up to one year prior to the assigned index date identified with the RxRisk-V model) and selected baseline comorbidities (ie, adjusted for diabetes at baseline for cardiovascular disorder; adjusted for cardiovascular disorder at baseline for diabetes and hyperlipidaemia). Stratified Cox regression was performed, with matched pairs as strata (to account for the confounding effect of age on comorbidities, as matching between cancer and control groups was undertaken with respect to age at index date). The proportional hazard assumption was assessed by examining whether the hazard ratio varied over time; for this purpose, interaction between study groups and follow-up time was included in the model. For five comorbidities (diabetes, gastric acid disorders, hyperlipidaemia, reactive airway diseases, pain or pain–inflammation), the hazard ratio did not vary with time. For three comorbidities (cardiovascular disorders, depression, osteoporosis), it varied with time, and separate hazard ratios were computed for each year since cohort entry. All analyses were performed in SAS 9.4 (SAS Institute).
Ethics approval
Ethics approval was not required for the analysis of de-identified PBS data.
Results
Data for 4278 women with hormone-dependent breast cancer who received endocrine therapy for the first time during 2004–2011 were analysed (Box 1). At least one dispensing record for a cardiovascular condition up to 12 months prior to initiation of endocrine therapy was present for 2756 women (64%); medications for pain or pain–inflammation (51%), gastric acid disorders (41%), hyperlipidaemia (36%) and depression (22%) were also frequently dispensed. Most women with hormone-dependent breast cancer were aged 55 years or more (range, 76–89%, depending on comorbidity; online Appendix, table 2).
Development of comorbidities
Therapies for six of the eight comorbidities of interest were initiated more frequently for women in the breast cancer group than for those in the control group: depression (overall hazard ratio [HR], 1.36), pain or pain–inflammation (HR, 1.30), osteoporosis (overall HR, 1.27), diabetes (HR, 1.24), cardiovascular disorders (overall HR, 1.22), and gastric acid disorders (HR, 1.20) (Box 2, Box 3, Box 4, Box 5). The exceptions were hyperlipidaemia (more frequent in the control group: HR, 0.88) and reactive airway diseases (no significant difference between the two groups: HR, 1.05) (Box 2).
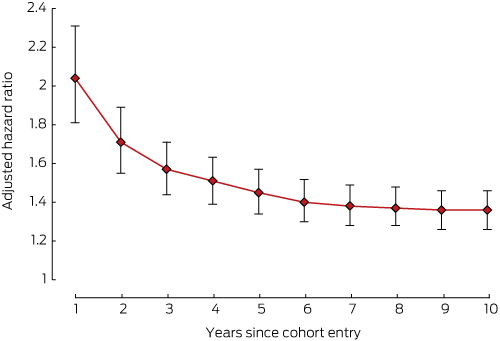
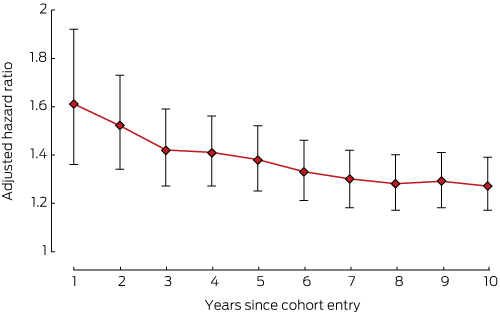
The risks of developing cardiovascular disorders (Box 3), depression (Box 4), or osteoporosis (Box 5) in the breast cancer cohort were all highest during the first year of therapy.
Discussion
This is the first Australian study to examine in a real world setting the relationship between a diagnosis of hormone-dependent breast cancer and the risk of developing a chronic disease. We investigated the comorbidity burden in women with hormone-dependent breast cancer at the commencement of endocrine therapy and its evolution over ten years. Cardiovascular disorders, depression, diabetes, gastric acid disorders, osteoporosis and pain or pain–inflammation developed more frequently in women with hormone-dependent cancer than in control groups of age-matched women. The relative risks for cardiovascular disorders, depression and osteoporosis in the cancer group were highest during the first year of endocrine therapy.
Our results are consistent with those of a study in the United Kingdom in which the development of common comorbidities by patients with breast cancer over a 7-year period were analysed; the authors found a higher prevalence of circulatory morbidities among patients with breast cancer than in the comparison population.15 Nonetheless, the ability to compare our findings with those of the UK study is limited by differences in the types and severity of conditions included; for example, the UK study included morbidities that resulted in admission to hospital, whereas we used medication dispensing records. In contrast to the UK study, we found that the incidence of digestive morbidities was significantly higher among women with hormone-dependent breast cancer than in women in the control group. The need for a medication to treat gastric acid symptoms may be related to previous exposure to corticosteroids as part of cancer chemotherapy or to another chemotherapy that predisposes women with breast cancer to gastrointestinal disorders.16 Our results are also consistent with other studies which found that depressive symptoms are more prevalent during the first 6–12 months after the diagnosis and initiation of treatment of cancer; the level of distress declined as women adjusted to the initial shock of their diagnosis and the acute adverse effects of therapy.17,18
There are several potential explanations for women with hormone-dependent breast cancer having an elevated risk of developing comorbidities. Cancer medications may contribute to the development of chronic conditions, including cardiovascular diseases (linked with anthracyclines, trastuzumab and radiation therapy),4,5 osteoporosis (aromatase inhibitors and cancer treatments that induce ovarian suppression),6,19 and musculoskeletal pain (aromatase inhibitors).20
Further, breast cancer shares risk factors with several chronic conditions. A higher risk of cardiovascular disease and diabetes among women with breast cancer, for instance, may be linked to excessive alcohol consumption, obesity and physical inactivity.21,22 The risk of breast cancer is increased by weight gain,23 and this may further increase the risk of cardiovascular and metabolic diseases.
The higher incidence of comorbidities may also reflect greater awareness and increased health care use associated with the treatment of breast cancer.15,24 This applies particularly to osteoporosis, as bone density testing is recommended before commencing aromatase inhibitor therapy,19 perhaps revealing previously undetected osteoporosis. The increased incidence of cardiovascular diseases during the first year of therapy may be linked to more intense monitoring of cardiac function or the early cardiotoxic effects of cancer treatment.4,5
In contrast, the incidence of hyperlipidaemia was lower among women with hormone-dependent breast cancer. This may reflect the effects of hormonal therapy; tamoxifen, for example, is reported to have favourable effects on the lipid profile of treated women.25,26 Aromatase inhibitors do not share this positive effect, but the lipid profiles of women receiving this agent are comparable with those of control women.25,26 There was no significant difference between the two groups in our study in the incidence of reactive airway diseases, which may indicate that breast cancer treatments are unlikely to increase the risk of developing respiratory conditions.
Our study has several limitations. Firstly, we used medicine dispensing as a surrogate measure for comorbidities, but there may be a time lag between the development of disease and the prescribing of medication. Chronic diseases such as diabetes and hyperlipidaemia, which may be controlled by diet alone, might be missed. Secondly, analgesics such as paracetamol and nonsteroidal anti-inflammatory drugs purchased over the counter are not captured in PBS data, and this may result in our underestimating the use of analgesics. Thirdly, the severity of comorbidities was not taken into account when assigning baseline comorbidity scores with the RxRisk-V model. We had no data on breast cancer stages, family history, body mass index, date of death, or other clinical factors or treatments, such as radiotherapy and chemotherapy; these aspects may themselves be associated with an increased risk of developing certain comorbidities. Endocrine therapy is subsidised by the PBS for both early and advanced hormone-dependent breast cancer (with the same PBS codes), with the exception of exemestane (indicated only for metastatic breast cancer); we were therefore unable to ascertain breast cancer stages from dispensing records. Our study population was restricted to concessional beneficiaries, who tend to be older and on lower incomes than general beneficiaries, but who also account for most medication use in Australia.27 People who did not use the health system (people who are healthy or did not consult prescribers and for whom there were consequently no dispensing records in the index year) were excluded from being selected in our control groups, perhaps leading to our overestimating the prevalence of comorbidities in the control groups.
In conclusion, our results indicate that the six chronic conditions we assessed are more likely to develop in women who have been diagnosed with hormone-dependent breast cancer than in women without cancer. As most women diagnosed with breast cancer in Australia can now be cured, the burden of non-cancer comorbidities is becoming the major health concern for these patients, but this is still largely unrecognised. Future breast cancer research should focus on strategies that effectively respond to the burden imposed by these comorbidities. Given the limitations of our single dataset study, promoting additional routine data capture and linkage in Australia would be valuable for obtaining a more comprehensive overview of the development of comorbidities in women with hormone-dependent breast cancer.
Box 1 – The numbers of women with hormone-dependent breast cancer in each comorbidity group, and of women in the corresponding control groups
|
Comorbidity |
Women with breast cancer |
Women in control group (10:1) |
|||||||||||||
|
Total number |
Excluded (pre-existing morbidity) |
Included in time-to-event analysis |
|||||||||||||
|
|
|||||||||||||||
|
Cardiovascular disorder |
4278 |
2756 (64%) |
1522 (36%) |
15 220 |
|||||||||||
|
Depression |
4278 |
934 (22%) |
3344 (78%) |
33 440 |
|||||||||||
|
Diabetes |
4278 |
479 (11%) |
3799 (89%) |
37 990 |
|||||||||||
|
Gastric acid disorder |
4278 |
1735 (41%) |
2543 (59%) |
25 430 |
|||||||||||
|
Hyperlipidaemia |
4278 |
1521 (36%) |
2757 (64%) |
27 570 |
|||||||||||
|
Osteoporosis |
4278 |
413 (10%) |
3865 (90%) |
38 650 |
|||||||||||
|
Reactive airway disease |
4278 |
817 (19%) |
3461 (81%) |
34 610 |
|||||||||||
|
Pain/pain–inflammation |
4278 |
2193 (51%) |
2085 (49%) |
20 850 |
|||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Ten-year incidence rates for comorbidities in women with hormone-dependent breast cancer and in women without cancer, with crude and adjusted hazard ratios
|
Comorbidity |
Incidence rate, per 100 person-years (95% CI)* |
Hazard ratio, cases v controls (95% CI) |
|||||||||||||
|
Cancer group (cases) |
Control group |
Crude |
Adjusted† |
||||||||||||
|
|
|||||||||||||||
|
Depression |
5.56 (5.20–5.94) |
4.10 (4.01–4.20) |
1.39 (1.29–1.49)‡ |
1.36 (1.26–1.46)‡ |
|||||||||||
|
Pain/pain–inflammation |
23.1 (22.0–24.4) |
17.9 (17.6–18.2) |
1.29 (1.22–1.37) |
1.30 (1.23–1.38) |
|||||||||||
|
Osteoporosis |
3.03 (2.80–3.29) |
2.41 (2.35–2.48) |
1.28 (1.17–1.40)‡ |
1.27 (1.17–1.39)‡ |
|||||||||||
|
Diabetes |
1.46 (1.30–1.64) |
1.17 (1.12–1.21) |
1.26 (1.11–1.42) |
1.24 (1.10–1.41) |
|||||||||||
|
Cardiovascular disorder |
13.2 (12.3–14.2) |
10.9 (10.6–11.1) |
1.23 (1.14–1.33)‡ |
1.22 (1.13–1.32)‡ |
|||||||||||
|
Gastric acid disorder |
10.8 (10.2–11.4) |
8.86 (8.69–9.04) |
1.21 (1.13–1.29) |
1.20 (1.13–1.28) |
|||||||||||
|
Reactive airway disease |
5.88 (5.52–6.27) |
5.43 (5.32–5.54) |
1.09 (1.02–1.17) |
1.05 (0.98–1.13) |
|||||||||||
|
Hyperlipidaemia |
5.13 (4.76–5.54) |
5.54 (5.42–5.67) |
0.91 (0.83–0.98) |
0.88 (0.81–0.96) |
|||||||||||
|
|
|||||||||||||||
|
* Case numbers are available in the online Appendix, table 3. † Adjusted for the number of comorbidities at baseline; for diabetes and hyperlipidaemia, also adjusted for cardiovascular conditions at baseline; for cardiovascular disorder, also adjusted for diabetes at baseline. ‡ Overall hazard ratio (hazard ratio varied over time: Box 3, Box 4, Box 5). |
|||||||||||||||
Box 3 – Adjusted hazard ratios (with 95% confidence intervals) for incidence of cardiovascular disorder, women with hormone-dependent breast cancer v women without cancer*
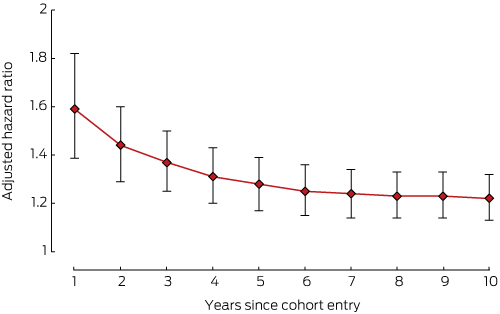
* Adjusted for diabetes and number of comorbidities at baseline.
Received 3 January 2017, accepted 18 May 2017
- Huah Shin Ng1
- Bogda Koczwara2,3
- David M Roder4
- Theo Niyonsenga1,5
- Agnes I Vitry1
- 1 University of South Australia, Adelaide, SA
- 2 Flinders Medical Centre, Adelaide, SA
- 3 Flinders Centre for Innovation in Cancer, Flinders University, Adelaide, SA
- 4 Centre for Population Health Research, University of South Australia, Adelaide, SA
- 5 Health Research Institute/CeRAPH, University of Canberra, Canberra, ACT
Huah Shin Ng is supported by an Australian Government Research Training Program Scholarship.
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Cancer in Australia: an overview 2014 (AIHW Cat. No. CAN 88; Cancer Series No. 90). Canberra: AIHW, 2014.
- 2. Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26: v8-v30.
- 3. Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 2014; 32: 2255-2269.
- 4. Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced cardiotoxicity. Mædica 2013; 8: 59-67.
- 5. Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol 2012; 23: vii155-vii66.
- 6. Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer 2011; 11: 384.
- 7. Owusu C, Buist DSM, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol 2008; 26: 549-555.
- 8. Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat 2011; 125: 191-200.
- 9. Pearson SA, Pesa N, Langton JM, et al. Studies using Australia’s Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: a systematic review of the published literature (1987–2013). Pharmacoepidemiol Drug Saf 2015; 24: 447-455.
- 10. Vitry A, Wong SA, Roughead EE, et al. Validity of medication-based co-morbidity indices in the Australian elderly population. Aust N Z Publ Health 2009; 33: 126-130.
- 11. Srasuebkul P, Dobbins TA, Pearson SA, Elements of Cancer Care (EoCC). Validation of a proxy for estrogen receptor status in breast cancer patients using dispensing data. Asia-Pac J Clin Oncol 2014; 10: E63-E68.
- 12. Sloan KL, Sales AE, Liu CF, et al. Construction and characteristics of the RxRisk-V — a VA-adapted pharmacy-based case-mix instrument. Med Care 2003; 41: 761-774.
- 13. Lu CY, Barratt J, Vitry A, Roughead E. Charlson and Rx-Risk comorbidity indices were predictive of mortality in the Australian health care setting. J Clin Epidemiol 2011; 64: 223-228.
- 14. Australian Institute of Health and Welfare. Chronic disease comorbidity [webpage]. Archived: https://web.archive.org/web/20170711172814/http://www.aihw.gov.au/chronic-diseases/comorbidity/ (viewed Mar 2017).
- 15. Macmillan Cancer Support, National Cancer Intelligence Network. Routes from diagnosis [website]. 2017. http://www.macmillan.org.uk/about-us/what-we-do/evidence/cancer-intelligence/routes-from-diagnosis.html (viewed Mar 2017).
- 16. Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases – a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016; 14: 179.
- 17. Bower JE. Behavioral symptoms in breast cancer patients and survivors: fatigue, insomnia, depression, and cognitive disturbance. J Clin Oncol 2008; 26: 768-777.
- 18. Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ 2005; 330: 702.
- 19. Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev 2008; 34: S3-S18.
- 20. Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor related arthralgia among breast cancer survivors. Cancer 2009; 115: 3631-3639.
- 21. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133: 1104-1114.
- 22. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010; 33: 1674-1685.
- 23. Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol 2005; 23: 774-782.
- 24. Danese MD, O’Malley C, Lindquist K, et al. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol 2011; 23: 1756-1765.
- 25. Filippatos TD, Liberopoulos EN, Pavlidis N, et al. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev 2009; 35: 175-184.
- 26. Gandhi S, Verma S. Aromatase inhibitors and cardiac toxicity: getting to the heart of the matter. Breast Cancer Res Treat 2007; 106: 1-9.
- 27. Australian Government Department of Health. Expenditure and prescriptions twelve months to 30 June 2016. The Pharmaceutical Benefits Scheme [website]; updated Dec 2016. http://www.pbs.gov.au/info/statistics/pbs-expenditure-prescriptions-30-june-2016 (viewed Oct 2017).





Abstract
Objective: To compare how frequently selected chronic diseases developed in women with breast cancer receiving endocrine therapy, and in women without cancer.
Design, setting and participants: Retrospective, rolling cohort study, analysing a random 10% sample of Pharmaceutical Benefits Scheme (PBS) data for the period 1 January 2003 – 31 December 2014. Women with breast cancer who first commenced endocrine therapy between January 2004 and December 2011 were identified, and age- and sex-matched (1:10) by comorbidity with control groups of women who did not have a dispensing record for antineoplastic agents during the study period or the comorbidity of interest at baseline.
Main outcome measures: Development of any of eight pre-selected comorbidities, identified in PBS claims data with the RxRisk-V model.
Results: Women with hormone-dependent breast cancer were significantly more likely than women in the control group to develop depression (overall hazard ratio [HR], 1.36; 95% CI, 1.26–1.46), pain or pain–inflammation (HR, 1.30; 95% CI, 1.23–1.38), osteoporosis (overall HR, 1.27; 95% CI, 1.17–1.39), diabetes (HR, 1.24; 95% CI, 1.10–1.41), cardiovascular disorders (overall HR, 1.22; 95% CI, 1.13–1.32), and gastric acid disorders (HR, 1.20; 95% CI, 1.13–1.28). The hazard ratios for developing cardiovascular disorders, depression and osteoporosis were highest during the first year of endocrine therapy. The risk of hyperlipidaemia was lower among women with breast cancer than in the control group (HR, 0.88; 95% CI, 0.81–0.96). There was no significant difference between the two groups in the risk of reactive airway diseases (HR, 1.05; 95% CI, 0.98–1.13).
Conclusion: Comorbid conditions are more likely to develop in women who have been diagnosed with hormone-dependent breast cancer than in women without cancer. Our results further support the need to develop appropriate models of care to manage the multiple chronic disorders of breast cancer survivors.