The known Sepsis is one the main causes of death in children worldwide. Childhood mortality in high income countries is higher in indigenous populations, but the contribution of infections to excess mortality in this vulnerable group is unknown.
The new Intensive care unit admission rates for invasive infections were higher for Aboriginal and/or Torres Strait Islander children than for non-Indigenous Australians, particularly for staphylococcal infections, and the population-based ICU mortality attributable to infections was more than twice that of non-Indigenous children.
The implications Our results highlight an important area of health care inequity in a high income country that must be redressed.
Of the estimated 6.3 million deaths of children under 5 years of age worldwide in 2013, 51.8% were attributable to infectious diseases.1,2 While most infection-related deaths occur in low income countries, infectious diseases also continue to cause significant mortality in high income countries.3 We have recently reported that the incidence of severe infections in children that require admission to an intensive care unit (ICU) was increasing in Australia and New Zealand,4 consistent with recent reports from the United States.5 About 25% of deaths in paediatric ICUs are related to severe infections, and case fatality rates are as high as 17%.4
In countries such as Canada, the US, Australia and New Zealand, indigenous status is a risk factor for childhood mortality because of a complex interplay of factors, including remoteness and access to health care, socio-economic and educational resources.6 In Australia, children of Aboriginal and Torres Strait Islander background are more than twice as likely to die during childhood than non-Indigenous Australian children.6 Indigenous Australian children have a higher risk of contracting infectious diseases, including otitis media and pyoderma, as well as invasive infections, including bloodstream infections, pneumonia and bronchiectasis;7-11 invasive infection rates are comparable with those in low and middle income countries. While the population-based incidence of sepsis, ICU admission, and mortality are reported to be significantly higher for Indigenous Australian adults,9 analogous data for Indigenous children have not been published.
In this article we report on severe infections in Indigenous children in Australia, using the previously published Australian and New Zealand paediatric sepsis study dataset.4 The aim of our study was to assess the epidemiology and mortality of severe infections in critically ill Indigenous Australian children, and to compare them with those for Australian children of non-Indigenous background.
Methods
We performed a multi-centre, retrospective national cohort study of Australian and New Zealand Paediatric Intensive Care (ANZPIC) Registry data (http://www.anzics.com.au/pages/CORE/ANZPICR-registry.aspx). The ANZPIC Registry is a state-funded mandatory registry that prospectively records data for paediatric admissions to all Australian and New Zealand paediatric and general ICUs, collected by trained ICU data collectors, with central data validation and auditing. Details of our study methods and use of registry data have been published previously.4 Only data from Australian units were included in the analysis reported in this article.
Our primary study group included Indigenous children less than 16 years of age who required non-elective admission to a paediatric or general ICU in Australia between 1 January 2002 and 31 December 2013. Indigenous status was defined by identification of the child as Aboriginal and/or Torres Strait Islander in the ANZPIC dataset. Non-Indigenous children under 16 years of age who required non-elective admission to an ICU in Australia during the same period served as a comparison group.
Infection groups were defined according to the diagnostic coding for each patient in the ANZPIC Registry, including the principal diagnosis, underlying diagnosis, and any of the associated diagnoses. “Invasive infection” included sepsis, septic shock, toxic shock, meningitis, pneumonia/pneumonitis, peritonitis, necrotising fasciitis, septic arthritis, osteomyelitis, endocarditis, tracheitis, and epiglottitis. We performed subgroup analyses of data for children with coded diagnoses of sepsis, septic shock or toxic shock. Sepsis and septic shock were defined according to the consensus definition.12
The primary outcome was ICU mortality. Severity of illness at ICU admission was assessed with the Paediatric Index of Mortality 2 (PIM2).13 Secondary outcomes included duration of invasive and non-invasive ventilation, length of stay in the ICU, length of stay in hospital, hospital mortality, and blood culture results.
The following comorbidities and underlying disease groups were defined: premature birth (earlier than 37 weeks’ gestation), congenital heart disease, chronic pulmonary disease, chronic renal disease, chronic neurological disease (encephalopathy, chronic central or peripheral nervous system disease), burns, and immunodeficiency or immunosuppression (with subgroups: haematological or solid organ tumour, status post-bone marrow transplantation, and solid organ transplant recipients).
Age-specific population data were obtained from the Australian Bureau of Statistics.14 Age-standardised admission rates were calculated with reference to the 2013 Indigenous population.15 Non-Indigenous admission rates were age-standardised with reference to 2013 non-Indigenous Australian population figures.14
Statistical analysis
Data are presented as numbers and percentages, or as means with standard deviations. Means for subgroups were compared in t tests. Admission rates were calculated for estimating population-based incidence and mortality rates. Rates were compared as incidence rate ratios, and proportions for Indigenous and non-Indigenous admissions were compared in exact binomial tests.
Analyses included every ICU admission. We compared differences between the first 6 years of the study (2002–2007: initiation of the “Surviving Sepsis Campaign”, after publication of the sepsis consensus definition), and the second 6 years of the study (2008–2013: period following publication of the second edition of the “Surviving Sepsis” guidelines16). In addition, trends during the 12-year study period were assessed by linear regression. We used the PIM2 score to adjust for the severity of illness of children. For risk prediction models, we used a saturated logistic regression model, clustering on site. For multivariable models, all significant predictors from the univariable analyses were included. We used a backward stepwise elimination procedure to eliminate non-significant predictors (P > 0.05). The severity of illness for Indigenous and non-Indigenous children admitted to an ICU were compared in a Wilcoxon rank-sum test.
We used the 2006 Australian Bureau of Statistics Remoteness Structure to classify each patient’s residential postcode as a major city, inner regional, outer regional, remote, or very remote Australia. Where postcodes contained different remoteness classifications, we assigned the classification applicable to the greater land area of the postcode.17
All analyses were conducted in Stata 12.1 (StataCorp). P < 0.05 was deemed statistically significant.
Ethics approval
The development of the research proposal was conducted in consultation with local Aboriginal Liaison officers and medical experts working with Aboriginal and Torres Strait Islander children in ICUs, and in accordance with national guidelines for ensuring cultural sensitivity. Approval was obtained from the Mater Health Services Aboriginal liaison officer. Ethics approval, including for waiver of informed consent, was obtained from the Mater Health Services Human Research Ethics Committee, Brisbane (reference, MHS HREC EC00332; HREC/13/MHS/153/AM02). The Registry was approved by the Human Research and Ethics Committee of the Royal Children’s Hospital (Brisbane).
Results
Study population
During 1 January 2002 – 31 December 2013, 82 750 children were admitted to a paediatric or general ICU in Australia; 4864 (5.9%) were of Indigenous (Aboriginal and/or Torres Strait Islander) background. Data for the 3150 Indigenous children who were non-electively admitted (65% of Indigenous patients) were included in our study. The proportion of children admitted to an ICU who were Indigenous Australians increased slightly but significantly over the study period, from 4.9% in 2002 to 6.3% of admissions in 2013 (0.13% per year; 95% confidence interval [CI], 0.01–0.17%; P = 0.037).
Invasive infections accounted for 23.0% of non-elective admissions of Indigenous children to ICUs (726 of 3150), compared with 17.3% for non-Indigenous children (8002 of 46 238; P < 0.001); 9.6% of Indigenous patients (303 of 3150) presented with severe sepsis or septic shock. The median age of Indigenous children at admission with invasive infections was 1.7 years (interquartile range [IQR], 0.4–6.7; range, 0–16 years), and 405 (55.8%) were boys. Overall, 39.7% of Indigenous patients with invasive infections had a major comorbidity. Detailed demographic and clinical data for patients with or without sepsis/septic shock are included in Box 1.
Admission rates
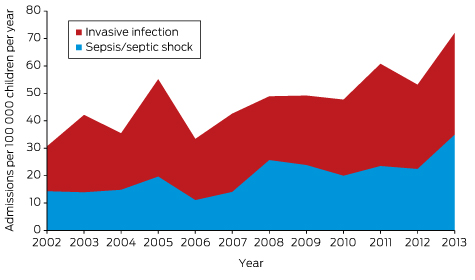
The estimated population-based age-standardised ICU admission rate for Indigenous children for all invasive infections was 47.6 per 100 000 children per year, rising from 30.7 in 2002 to 72.0 per 100 000 in 2013; the mean annual increase in this rate was 2.61 per 100 000 children per year (95% CI, 1.20–4.02; P = 0.002). The population-based admission rate for sepsis/septic shock was estimated to be 19.8 per 100 000 children per year, rising from 14.3 in 2002 to 34.8 per 100 000 in 2013; the mean annual increase in this rate was 1.45 per 100 000 children per year (95% CI, 0.62–2.28; P = 0.003; Box 2, Appendix 1).
The estimated population-based admission rate of non-Indigenous patients for all invasive infections was 15.9 per 100 000 children per year (annual increase, 0.67 per 100 000 children per year; 95% CI, 0.42–0.90; P < 0.001). The population-based admission rate for sepsis/septic shock was estimated to be 6.8 per 100 000 children per year (annual increase, 0.15 per 100 000 children per year; 95% CI, 0.05–0.26; P = 0.009).
Infection focus and pathogens
The most frequent invasive infectious conditions in Indigenous patients were pneumonia (394 of 726, 54.3%), meningitis (89 of 726, 12.3%), peritonitis (22 of 726, 3.0%), and osteomyelitis (16 of 726, 2.2%). The main pathogens identified in critically ill Indigenous children admitted with sepsis/septic shock were Staphylococcus aureus (22% of patients) and Neisseria meningitidis (11%; Box 3). A bacterial aetiology was identified in 54% of patients and a viral co-infection was reported in 11% of cases. No pathogen was recorded for 38% of episodes of sepsis/septic shock. The proportion of patients diagnosed with N. meningitidis infection decreased from 16% in 2002–2007 to 8% of those with sepsis/septic shock during 2008–2013 (P = 0.044), but no substantial changes in the frequency of infection with other pathogens were observed.
The estimated population-based S. aureus sepsis admission rate was 4.42 per 100 000 children per year for Indigenous children and 0.57 per 100 000 children per year for non-Indigenous children (rate ratio, 7.7; 95% CI, 5.8–10.1; P < 0.001).
Outcomes
The mean ICU and hospital lengths of stay for Indigenous children with an invasive infection were 5.8 and 26.9 days respectively. The crude ICU mortality for all invasive infections in Indigenous children was 5.6% (41 of 726), and 8.3% among those with sepsis/septic shock (25 of 303); the rates for non-Indigenous children were 6.5% and 10.2% respectively (data not shown). The predicted risk of death for non-Indigenous children (PIM2 score) was significantly lower in 2008–2013 than in 2002–2007, but there was no clinically or statistically significant decline in scores for Indigenous children (Appendix 2). After adjusting for PIM2 score in logistic regression, there was no significant difference in mortality between Indigenous and non-Indigenous children (odds ratio, 0.75; 95% CI, 0.53–1.07; P = 0.12). Invasive infections were involved in 29.3% of deaths of Indigenous children with a non-elective ICU admission. We estimated a population-based age-standardised ICU mortality rate for invasive infections in Indigenous children of 2.67 per 100 000 children per year, compared with 1.04 per 100 000 children per year for non-Indigenous children (rate ratio, 2.65; 95% CI, 1.88–3.64; P < 0.001).
Univariate logistic regression models (Box 4, Appendix 3) identified that a comorbidity (in particular, immunosuppression or chronic neurological disease) and severity markers (including need for mechanical ventilation, extracorporeal membrane oxygenation, and renal replacement) were significant predictors of mortality of Indigenous children with invasive infections. In multivariate analyses, immunosuppression, chronic neurological disease, and need for mechanical ventilation or renal replacement remained significantly associated with mortality; the strongest effect size was associated with the need for mechanical ventilation. Similar findings were made for children with sepsis, except that congenital heart disease, rather than immunosuppression or neurological disease, was a significant predictor of mortality in both uni- and multivariate analyses.
Discussion
This is the largest study to have examined life-threatening infections in Indigenous Australian children, exploring an important area of health inequity in a high income country. Our population-based study of more than 3000 Indigenous children admitted to ICUs found a disproportionately high burden of disease associated with invasive infections in Aboriginal and Torres Strait Islander children, causing significant excess childhood mortality. In contrast to previous studies that focused on mild to moderate infections, our study examined severe, life-threatening infections. During 2002–2013, the ICU admission rate for invasive infections was three times as high for Indigenous as for non-Indigenous children (47.6 v 15.9 per 100 000 children per year); the estimated population-based ICU mortality attributable to invasive infections was more than double that for non-Indigenous children.
Our findings parallel reports of disproportionate infectious disease burdens in other indigenous populations.6,8,18-20 High rates of infectious diseases, including ear and respiratory tract infections, have consistently been reported for Canadian First Nations, Inuit and Métis peoples, Native Americans, Alaskan and Hawaiian Natives, and New Zealand Pacific and Māori peoples. Children in these populations experience poorer overall health outcomes than non-indigenous children, including higher infant and childhood mortality.6 Risk factors that contribute to the high incidence of infectious diseases among indigenous children include lower socio-economic status, overcrowding, poor access to sanitation, clean water and health facilities, and differences in hygiene and health-seeking behaviour.21 These key social and economic factors, recognised as major causes of poor health in low income countries, must also receive attention in high income countries if we are to improve national and global health outcomes for disadvantaged groups.22
Remoteness and difficulties in access to health care may also significantly increase the incidence of invasive infections in indigenous children that require treatment in ICUs, as delays in initiating appropriate treatment allow disease progression.23 This is consistent with our finding that Indigenous Australian children admitted to ICUs with invasive infections had higher median illness severity scores (PIM2) than non-Indigenous children during 2008–2013. This finding also suggests that a lower admission threshold for Indigenous children is unlikely to have contributed to their higher admission rate in our study. Further investigations in which outcomes are stratified according to the length of time to medical care may clarify the impact of remoteness on outcomes for children with infectious diseases.
The incidence of pneumococcal, group A streptococcal, and meningococcal disease in Indigenous Australian children has been reported to be 2–10 times that for non-Indigenous children residing in the same geographical area, despite 7-valent pneumococcal and meningococcal C vaccinations.20,24,25 We measured a significant decrease in meningococcal sepsis among Indigenous children that coincided with the introduction of the conjugate meningococcal vaccine. While expanding targeted vaccination may lead to further reductions in bacterial sepsis rates, vaccine-based interventions are unlikely to be available soon for S. aureus, identified in 40% of Indigenous patients from whom bacteria were isolated in our study. In the overall Australian and New Zealand paediatric sepsis ICU study,4 only 10% of sepsis cases were attributable to S. aureus, comparable with other cohorts.26 Prospective studies that assess the continuing impact of vaccinations on severe infection rates among Indigenous children in Australia are needed to further define this important area of public health.
Very high rates of invasive staphylococcal infection have been reported in adult and paediatric Indigenous populations in Australia.11 Excess rates of S. aureus infection have also been documented among Māori, Pacific Islanders and Samoans in New Zealand, Canadian Aboriginals, Alaskan Natives, Pacific Islanders in Hawaii, and Native Americans. Miles and colleagues27 found that 81% of patients admitted to paediatric ICUs in New Zealand for invasive S. aureus infections were Pacific Island and Māori children, although these ethnic groups comprise only 22% of the population. It is possible that genetic susceptibility may contribute to differences in biological susceptibility to sepsis,21 but further research is needed to clarify this question.
A number of limitations of our study need to be considered. It was based on a prospective paediatric ICU registry that does not capture infectious disease outcomes for newborns in neonatal nurseries or for children who die before they can be admitted to an ICU. The ANZPIC Registry captures 92–94% of paediatric patients admitted to ICUs in Australia, and the number of general ICUs contributing data to the registry increased during the study period. Admissions to general ICUs represented 17.1% of non-elective admissions recorded by this registry during 2008–2013, and 9.4% of admissions during 2002–2007. We therefore cannot exclude the possibility that we underestimated the true population-based incidence of severe infections.
Our primary outcome measure was ICU mortality. We did not detect major changes over time in mortality for Indigenous children admitted to ICUs with severe infections, but our study was not powered for subgroup mortality analyses. It is noteworthy that using mortality as an endpoint does not capture the impact of ICU admission on quality of life and health after discharge; clinically significant short and long term effects in former paediatric ICU patients have been documented.27,28 Finally, Indigenous children in Australia live in diverse environments, ranging from cities to remote Indigenous communities. Our study did not stratify outcomes according to these potentially important differences, nor did we have access to data on socio-economic status.
Conclusion
Children of Aboriginal and Torres Strait Islander background were three times more likely to be admitted to an ICU for severe infections than non-Indigenous children during 2002–2013, and the population-based mortality attributable to infections in Indigenous children was more than twice that for non-Indigenous children. Our study highlights an important area of inequality in health care for Indigenous children that requires urgent attention. Further research is needed to define risk factors and to develop and assess appropriately targeted interventions.
Box 1 – Baseline characteristics and severity of disease at admission for Indigenous children admitted to an intensive care unit (ICU) with an invasive infection
Invasive infection without sepsis/septic shock |
Invasive infection with sepsis/septic shock |
||||||||||||||
Number of children |
423 |
303 |
|||||||||||||
Age of child |
|||||||||||||||
Median (IQR), years |
1.6 (0.4–5.5) |
1.9 (0.4–8.5) |
|||||||||||||
Neonates (< 28 days old) |
8 (1.9%) |
19 (6.3%) |
|||||||||||||
Infants (28–364 days old) |
162 (38.3%) |
95 (31.4%) |
|||||||||||||
1–4 years old |
138 (32.6%) |
88 (29.0%) |
|||||||||||||
5–9 years old |
59 (14.0%) |
43 (14.2%) |
|||||||||||||
10–15 years old |
56 (13.2%) |
58 (19.1%) |
|||||||||||||
Sex |
|||||||||||||||
Boys |
235 (55.6%) |
170 (56.1%) |
|||||||||||||
Admission category |
|||||||||||||||
Direct paediatric ICU admission* |
284 (67.1%) |
219 (72.3%) |
|||||||||||||
Inter-hospital transport |
180 (42.6%) |
140 (46.2%) |
|||||||||||||
Risk category |
|||||||||||||||
Any comorbidity |
148 (35.0%) |
140 (46.2%) |
|||||||||||||
Immunodeficiency or immunosuppression† |
|||||||||||||||
Oncology‡ |
6 (1.4%) |
19 (6.3%) |
|||||||||||||
Bone marrow transplantation |
4 (0.9%) |
2 (0.6%) |
|||||||||||||
Transplantation‡ |
0 |
1 (0.3%) |
|||||||||||||
Chronic neurological disease |
42 (9.9%) |
18 (5.9%) |
|||||||||||||
Chronic respiratory disease |
38 (9.0%) |
14 (4.6%) |
|||||||||||||
Congenital heart disease |
13 (3.1%) |
17 (5.6%) |
|||||||||||||
Premature birth |
27 (6.4%) |
30 (9.9%) |
|||||||||||||
Burns |
6 (1.4%) |
10 (3.3%) |
|||||||||||||
Chronic renal failure |
4 (0.9%) |
1 (0.3%) |
|||||||||||||
Severity |
|||||||||||||||
Mean ICU length of stay (SD), days |
5.2 (9.6) |
6.8 (8.3) |
|||||||||||||
Mechanical ventilation in the first hour |
222 (52.5%) |
161 (53.1%) |
|||||||||||||
Intubation and ventilation |
256 (60.5%) |
215 (71.0%) |
|||||||||||||
Mean PIM2 score (range) |
5.0% (0.2–95.6%) |
8.4% (0.2–99.5%) |
|||||||||||||
Extracorporeal membrane oxygenation |
1 (0.2%) |
7 (2.3%) |
|||||||||||||
Acute respiratory distress syndrome |
7 (1.7%) |
14 (4.6%) |
|||||||||||||
Renal replacement therapy |
4 (0.9%) |
11 (3.6%) |
|||||||||||||
PIM2 = Paediatric Index of Mortality 2 (mean probability of death). * Other admissions were to mixed ICUs. † Including primary immunodeficiency and secondary immunodeficiency (oncology, bone marrow transplantation, other transplantation, other immunosuppression). ‡ Excluding bone marrow transplantation. | |||||||||||||||
Box 2 – Age-standardised admission rate of Indigenous children to intensive care units for all invasive infections
Box 3 – Identified pathogens in Indigenous children admitted to an intensive care unit with sepsis or septic shock
|
Year of admission |
|||||||||||||||
2002–2007 |
2008–2013 |
P* |
2002–2013 |
||||||||||||
Number of patients |
108 |
195 |
303 |
||||||||||||
Bacterial |
|||||||||||||||
Staphylococcus aureus (methicillin-sensitive or -resistant) |
21 (19%) |
46 (24%) |
0.40 |
67 (22%) |
|||||||||||
Neisseria meningitidis |
17 (16%) |
16 (8%) |
0.044 |
33 (11%) |
|||||||||||
Streptococcus pneumoniae |
2 (2%) |
9 (5%) |
0.22 |
11 (4%) |
|||||||||||
Coagulase-negative Staphylococcus |
0 |
2 (1%) |
0.29 |
2 (1%) |
|||||||||||
Group A Streptococcus, Streptococcus viridans |
6 (6%) |
11 (6%) |
0.98 |
17 (6%) |
|||||||||||
Group B Streptococcus |
0 |
1 (1%) |
0.46 |
1 (< 1%) |
|||||||||||
Escherichia coli |
4 (4%) |
4 (2%) |
0.39 |
8 (3%) |
|||||||||||
Pseudomonas aeruginosa |
4 (4%) |
4 (2%) |
0.39 |
8 (3%) |
|||||||||||
Klebsiella spp. |
1 (1%) |
6 (3%) |
0.23 |
7 (2%) |
|||||||||||
Haemophilus influenzae type B |
2 (2%) |
0 |
0.057 |
2 (1%) |
|||||||||||
Other bacteria |
8 (7%) |
25 (13%) |
0.15 |
33 (11%) |
|||||||||||
Any bacteria |
56 (52%) |
108 (55%) |
0.55 |
164 (54%) |
|||||||||||
Fungal |
|||||||||||||||
Candida, Aspergillus spp., other fungus |
5 (5%) |
13 (7%) |
0.47 |
18 (6%) |
|||||||||||
Viral co-infection |
|||||||||||||||
Influenza |
2 (2%) |
2 (1%) |
0.55 |
4 (1%) |
|||||||||||
Parainfluenza |
0 |
2 (1%) |
0.29 |
2 (1%) |
|||||||||||
Respiratory syncytial virus |
2 (2%) |
7 (4%) |
0.39 |
9 (3%) |
|||||||||||
Adenovirus |
1 (1%) |
7 (4%) |
0.17 |
8 (3%) |
|||||||||||
Cytomegalovirus, Epstein–Barr, herpes simplex, varicella zoster viruses |
1 (1%) |
5 (3%) |
0.33 |
6 (2%) |
|||||||||||
Enterovirus |
0 |
2 (1%) |
0.29 |
2 (1%) |
|||||||||||
Other viruses |
1 (1%) |
3 (2%) |
0.66 |
4 (1%) |
|||||||||||
Any viral co-infection |
7 (6%) |
26 (13%) |
0.067 |
33 (11%) |
|||||||||||
No bacterial, fungal or viral organism identified |
47 (44%) |
67 (34%) |
0.12 |
114 (38%) |
|||||||||||
* 2002–2007 v 2008–2013. | |||||||||||||||
Box 4 – Summary of uni- and multivariate models for mortality associated with invasive infections in Indigenous (Aboriginal and Torres Strait Islander) children requiring intensive care unit (ICU) admission*
A. All invasive infectionsPatients |
Deaths |
Univariable analysis |
Multivariable analysis |
||||||||||||
Odds ratio |
P |
Odds ratio |
P |
||||||||||||
Total invasive infection cases |
726 |
41 (5.6%) |
|||||||||||||
Remoteness: No significant differences |
|||||||||||||||
Age† |
|||||||||||||||
5–9 years old |
102 (14.1%) |
11 (27%) |
2.70 (1.13–6.45) |
0.025 |
|||||||||||
Risk category |
|||||||||||||||
Immunodeficiency or immunosuppression |
33 (4.6%) |
6 (15%) |
4.18 (1.62–10.8) |
0.003 |
4.94 (1.59–15.4) |
0.006 |
|||||||||
Oncology‡ |
25 (3.4%) |
2 (4.9%) |
1.48 (0.34–6.49) |
0.61 |
|||||||||||
Bone marrow transplantation |
6 (0.8%) |
2 (4.9%) |
8.73 (1.55–49.1) |
0.014 |
|||||||||||
Solid organ transplant‡ |
1 (0.1%) |
0 |
— |
||||||||||||
Chronic neurological condition |
60 (8.3%) |
7 (17%) |
2.46 (1.04–5.80) |
0.041 |
2.58 (1.05–6.33) |
0.039 |
|||||||||
Any comorbidity |
288 (39.7%) |
25 (61%) |
2.51 (1.31–4.78) |
0.005 |
— |
||||||||||
ICU size: No significant differences |
|||||||||||||||
Admission category: No significant differences |
|||||||||||||||
Time period |
|||||||||||||||
2002–2007 |
295 (40.6%) |
14 (34%) |
Reference |
||||||||||||
2008–2013 |
431 (59.4%) |
27 (66%) |
1.34 (0.69–2.60) |
0.38 |
|||||||||||
Severity |
|||||||||||||||
Intubation and mechanical ventilation |
471 (64.9%) |
40 (98%) |
23.6 (3.22–172) |
0.002 |
21.5 (2.88–160) |
0.003 |
|||||||||
Extracorporeal membrane oxygenation |
8 (1.1%) |
3 (7.3%) |
10.7 (2.47–46.6) |
0.002 |
2.81 (0.45–17.4) |
0.27 |
|||||||||
Acute respiratory distress syndrome |
21 (2.9%) |
4 (9.8%) |
4.25 (1.36–13.3) |
0.013 |
1.37 (0.34–5.49) |
0.66 |
|||||||||
Renal replacement therapy |
15 (2.1%) |
6 (15%) |
12.9 (4.34–38.2) |
< 0.001 |
5.32 (1.45–19.5) |
0.012 |
|||||||||
Patients |
Deaths |
Univariable analysis |
Multivariable analysis |
||||||||||||
Odds ratio |
P |
Odds ratio |
P |
||||||||||||
Total non-elective sepsis and septic shock cases |
303 |
25 (8.3%) |
|||||||||||||
Remoteness: No significant differences |
|||||||||||||||
Age: No significant differences |
|||||||||||||||
Risk category |
|||||||||||||||
Congenital heart disease |
17 (5.6%) |
4 (16%) |
3.88 (1.16–13.0) |
0.027 |
4.39 (1.23–15.64) |
0.023 |
|||||||||
ICU size: No significant differences |
|||||||||||||||
Admission category: No significant differences |
|||||||||||||||
Time period |
|||||||||||||||
2002–2007 |
108 (35.6%) |
6 (24%) |
Reference |
||||||||||||
2008–2013 |
195 (64.4%) |
19 (76%) |
1.84 (0.71–4.74) |
0.21 |
|||||||||||
Severity |
|||||||||||||||
Intubation and mechanical ventilation |
215 (71.0%) |
24 (96%) |
10.9 (1.46–82.1) |
0.020 |
8.04 (1.05–61.6) |
0.045 |
|||||||||
Extracorporeal membrane oxygenation |
7 (2.3%) |
3 (12%) |
9.34 (1.97–44.4) |
0.005 |
1.97 (0.31–12.6) |
0.48 |
|||||||||
Acute respiratory distress syndrome |
14 (4.6%) |
3 (12%) |
3.31 (0.86–12.8) |
0.082 |
1.73 (0.37–8.18) |
0.49 |
|||||||||
Renal replacement therapy |
11 (3.6%) |
5 (20%) |
11.3 (3.18–40.4) |
< 0.001 |
6.77 (1.52–30.0) |
0.012 |
|||||||||
ICU = intensive care unit. * Full results of the analyses are included in Appendix 3. † Reference: infants (28–364 days old); ‡ Excluding bone marrow transplantation. | |||||||||||||||
Received 18 May 2016, accepted 8 August 2016
- Justyna A Ostrowski1,2
- Graeme MacLaren3,4,5
- Janet Alexander6
- Penny Stewart7
- Sheena Gune7
- Joshua R Francis8,9
- Subodh Ganu10
- Marino Festa11
- Simon J Erickson12
- Lahn Straney13
- Luregn J Schlapbach1,2,14
- 1 Mater Research Institute, University of Queensland, Brisbane, QLD
- 2 Lady Cilento Children's Hospital, Brisbane, QLD
- 3 University of Melbourne, Melbourne, VIC
- 4 Royal Children's Hospital, Melbourne, VIC
- 5 National University Health System, Singapore, Singapore
- 6 Australian and New Zealand Paediatric Intensive Care Registry (CORE), Brisbane, QLD
- 7 Alice Springs Hospital, Alice Springs, NT
- 8 Royal Darwin Hospital, Darwin, NT
- 9 Menzies School of Health Research, Charles Darwin University, Darwin, NT
- 10 Women's and Children's Hospital Adelaide, Adelaide, SA
- 11 Children's Hospital at Westmead, Sydney, NSW
- 12 Princess Margaret Hospital for Children, Perth, WA
- 13 Monash University, Melbourne, VIC
- 14 Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
This study was supported by the National Health and Medical Research Council (NHMRC) Australian Resuscitation Outcomes Consortium (Aus-ROC; ) Centre of Research Excellence (1029983). We thank the Aboriginal and Torres Strait Islander liaison officers at Mater Health Services, Brisbane, for critically reviewing the study protocol and manuscript. We thank the Paediatric Study Group of the Australian and New Zealand Intensive Care Society for supporting this study (Simon Erickson, Princess Margaret Hospital for Children, Perth; Andreas Schibler, Anthony Slater, Debbie Long, Jan Alexander, Lady Cilento Children’s Hospital, Brisbane; John Beca, Claire Sherring, Starship Children’s Hospital, Auckland, New Zealand; Gary Williams, Janelle Young, Mary Lou Morritt, Sydney Children’s Hospital, Sydney; Johnny Millar, Carmen Del Zoppo, Warwick Butt, Royal Children’s Hospital, Melbourne; Subodh Ganu, Georgia Letton, Women’s and Children’s Hospital, Adelaide; Marino Festa, Westmead Children’s Hospital, Sydney). We thank the intensivists, data managers and other staff in the participating intensive care units for contributing data. The ANZPIC Registry is supported by the Australian and New Zealand Intensive Care Society, the Ministry of Health (New Zealand), and state and territory health departments through the Australian Health Ministers’ Advisory Council.
Lahn Straney receives salary support from the NHMRC Aus-ROC Centre of Research Excellence (1029983).
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385: 430-440.
- 2. Kissoon N, Uyeki TM. Sepsis and the global burden of disease in children. JAMA Pediatr 2016; 170: 107-108.
- 3. Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in US children’s hospitals. Pediatr Crit Care Med 2014; 15: 798-805.
- 4. Schlapbach LJ, Straney L, Alexander J, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15: 46-54.
- 5. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Critical Care Med 2013; 14: 686-693.
- 6. Smylie J, Adomako P (editors). Indigenous children’s health report: health assessment in action. Toronto: St. Michael’s Hospital, 2009. http://www.stmichaelshospital.com/crich/wp-content/uploads/ichr_report-web.pdf (accessed Oct 2016).
- 7. O’Grady KF, Carlin JB, Chang AB, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against radiologically diagnosed pneumonia in indigenous infants in Australia. Bull World Health Organ 2010; 88: 139-146.
- 8. Singleton RJ, Valery PC, Morris P, et al. Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol 2014; 49: 189-200.
- 9. Davis JS, Cheng AC, McMillan M, et al. Sepsis in the tropical Top End of Australia’s Northern Territory: disease burden and impact on Indigenous Australians. Med J Aust 2011; 194: 519-524. <MJA full text>
- 10. Turnidge JD. High burden of staphylococcal disease in indigenous communities. J Infect Dis 2009; 199: 1416-1418.
- 11. Tong SY, Bishop EJ, Lilliebridge RA, et al. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J Infect Dis 2009; 199: 1461-1470.
- 12. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 2-8.
- 13. Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29: 278-285.
- 14. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, Jun 2012. Dec 2012. http://www.abs.gov.au/AUSSTATS/abs@.nsf/second+level+view?ReadForm&prodno=3101.0&viewtitle=Australian Demographic Statistics∼Jun 2012∼Previous∼18/12/2012&&tabname=Past Future Issues&prodno=3101.0&issue=Jun 2012&num=&view=& (accessed June 2016).
- 15. Australian Bureau of Statistics. 3238.0. Estimates and projections, Aboriginal and Torres Strait Islander Australians, 2001 to 2026. Apr 2014. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3238.02001 to 2026?OpenDocument (accessed June 2016).
- 16. Society of Critical Care Medicine. Surviving Sepsis Campaign: history [website]. http://www.survivingsepsis.org/About-SSC/Pages/History.aspx (accessed June 2016).
- 17. Australian Bureau of Statistics. Remoteness Structure [website]. Updated June 2014. http://www.abs.gov.au/websitedbs/D3310114.nsf/home/remoteness+structure (accessed June 2016).
- 18. Dalloo A, Sobol I, Palacios C, et al. Investigation of community-associated methicillin-resistant Staphylococcus aureus in a remote northern community, Nunavut, Canada. Can Commun Dis Rep 2008; 34: 1-7.
- 19. Einsiedel L, Fernandes L, Joseph S, et al. Non-communicable diseases, infection and survival in a retrospective cohort of Indigenous and non-Indigenous adults in central Australia. BMJ Open 2013; 3: e003070.
- 20. Moore HC, Lehmann D, de Klerk N, et al. Reduction in disparity for pneumonia hospitalisations between Australian Indigenous and non-Indigenous children. J Epidemiol Community Health 2012; 66: 489-494.
- 21. Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med 2008; 177: 279-284.
- 22. Marmot M. The health gap: the challenge of an unequal world. Lancet 2015; 386: 2442-2444.
- 23. Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014; 42: 2409-2417.
- 24. Whitehead BD, Smith HV, Nourse C. Invasive group A streptococcal disease in children in Queensland. Epidemiol Infect 2011; 139: 623-628.
- 25. Gear RJ, Carter JC, Carapetis JR, et al. Changes in the clinical and epidemiological features of group A streptococcal bacteraemia in Australia’s Northern Territory. Trop Med Int Health 2015; 20: 40-47.
- 26. Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15: 828-838.
- 27. Miles F, Voss L, Segedin E, Anderson BJ. Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch Dis Child 2005; 90: 1274-1278.
- 28. Alievi PT, Carvalho PR, Trotta EA, Mombelli Filho R. The impact of admission to a pediatric intensive care unit assessed by means of global and cognitive performance scales. J Pediatr (Rio J) 2007; 83: 505-511.





Abstract
Objectives: To describe the incidence and mortality of invasive infections in Indigenous children admitted to paediatric and general intensive care units (ICUs) in Australia.
Design: Retrospective multi-centre cohort study of Australian and New Zealand Paediatric Intensive Care Registry data.
Participants: All children under 16 years of age admitted to an ICU in Australia, 1 January 2002 – 31 December 2013. Indigenous children were defined as those identified as Aboriginal and/or Torres Strait Islander in a mandatory admissions dataset.
Main outcomes: Population-based ICU mortality and admission rates.
Results: Invasive infections accounted for 23.0% of non-elective ICU admissions of Indigenous children (726 of 3150), resulting in an admission rate of 47.6 per 100 000 children per year. Staphylococcus aureus was the leading pathogen identified in children with sepsis/septic shock (incidence, 4.42 per 100 000 Indigenous children per year; 0.57 per 100 000 non-Indigenous children per year; incidence rate ratio 7.7; 95% CI, 5.8–10.1; P < 0.001). While crude and risk-adjusted ICU mortality related to invasive infections was not significantly different for Indigenous and non-Indigenous children (odds ratio, 0.75; 95% CI, 0.53–1.07; P = 0.12), the estimated population-based age-standardised mortality rate for invasive infections was significantly higher for Indigenous children (2.67 per 100 000 per year v 1.04 per 100 000 per year; crude incidence rate ratio, 2.65; 95% CI, 1.88–3.64; P < 0.001).
Conclusions: The ICU admission rate for severe infections was several times higher for Indigenous than for non-Indigenous children, particularly for S. aureus infections. While ICU case fatality rates were similar, the population-based mortality was more than twice as high for Indigenous children. Our study highlights an important area of inequality in health care for Indigenous children in a high income country that needs urgent attention.